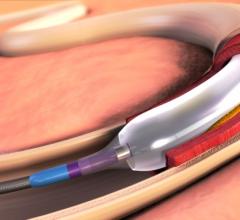
A lengthy review of increasing medical evidence shows that cancer treatments such as chemotherapy and radiation often damage heart vessels and tissues, increasing the risk of later heart disease in cancer survivors. Now an interventional cardiologist from Detroit Medical Center (DMC) who is a co-author of the study is helping efforts to expand a new medical specialty known as “cardioncology.”
The U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel is set to review data and offer recommendations concerning the final approval of Abbott’s Absorb fully bioresorbable stent and the AngelMed Guardian System.
A group of researchers from Russia, Australia and the Netherlands have developed a technology that can reduce magnetic resonance imaging (MRI) scanning times by more than 50 percent.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Bradycardia – a slower than normal heartbeat – does not increase the risk of developing cardiovascular disease, according to a study conducted by researchers at Wake Forest Baptist Medical Center. The study is published in the Jan.19 online edition of the Journal of American Medical Association Internal Medicine.
Global Genomics Group (G3) and GNS Healthcare (GNS), announced preliminary results of the GLOBAL Clinical Study designed to identify biomarkers associated with coronary artery disease (CAD). The study provided proof of concept of the approach by prospectively identifying a biochemical pathway known to be associated with CAD.
Vivasure Medical announced Conformité Européenne (CE) Mark approval of the world’s first fully bioabsorbable percutaneous vascular closure device for large-bore femoral arteriotomies.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Medtronic plc announced that the IN.PACT Admiral drug eluting balloon (DEB) has received CE (Conformité Européene) Mark for arteriovenous (AV) access to help maintain hemodialysis access in patients with end-stage renal disease.
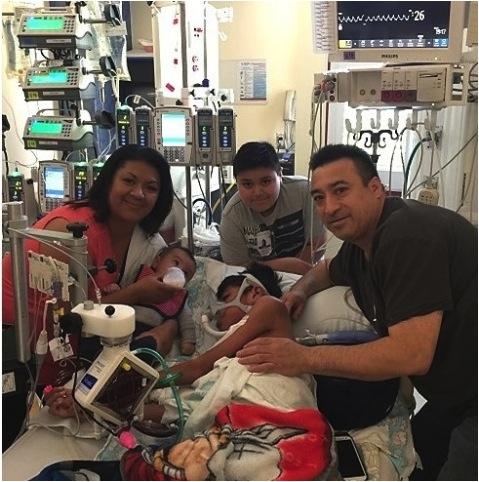
2016 is starting off a whole lot better than last year for 14-year-old Oswaldo Jimenez of Salem, Ore. Last year at this time, he was sick. Life-threateningly sick. Diagnosed with pulmonary arterial hypertension at age 9, his heart and lungs were failing, and a heart-lung transplant seemed to be his only real hope for survival.
Biointegrated sensors for long-term, continuous tracking of body chemistry may make health and disease monitoring as easy as turning on your smartphone. Unveiled by Profusa Inc., of South San Francisco, Calif., tiny bioengineered biosensors will soon enable real-time detection of our body's unique chemistry, providing actionable, medical-grade data for personal and medical use for as long as two years at a time. The company's technology and vision were presented at the CES Digital Health Summit, Jan. 6-9 in Las Vegas.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Teleflex Inc. has acquired privately held Nostix LLC, developer of innovative tip confirmation systems that are used to increase the accuracy of vascular access device placement.
Patients between the ages of 40 and 70 who undergo aortic valve replacement (AVR) may fare better with tissue-based valves than metal-based valves, according to a review article posted online by The Annals of Thoracic Surgery.
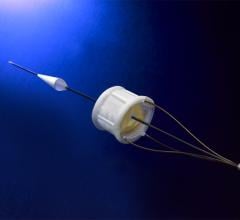
CardioKinetix Inc. announced that it has enrolled more than half of the subjects in its pivotal United States trial, PARACHUTE IV. The trial is evaluating the Parachute device for the treatment of patients suffering from heart failure, a highly debilitating condition.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The Centers for Medicare and Medicaid Services (CMS) announced Monday that it will be ending the Meaningful Use program that was meant to encourage the adoption of electronic medical records (EMR).

January 15, 2016 — The U.S. Food and Drug Administration (FDA) has given its approval for an expanded indication study ...
January 14, 2016 — Cornell biomedical engineers have discovered natural triggers that could reduce the chance of life ...


 January 20, 2016
January 20, 2016