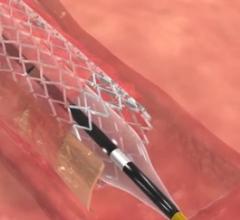
The following are the late-breaking clinical trial presentations presented at the 2016 American College of Cardiology ...
February 9, 2016 — Boston Scientific Corp. announced the Centers for Medicare and Medicaid Services (CMS) will cover ...
Off-label use of the St. Jude Amplatzer vascular plug devices offers a new solution for the minimally invasive repair of ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
February 8, 2016 — A parent’s incarceration has immediate, devastating effects on a family. Now, Virginia Tech and ...
MedStar Heart & Vascular Institute at MedStar Washington Hospital Center is one of two hospitals in the nation selected to partner with the American College of Cardiology (ACC) on the Find Your Heart a Home pilot program.
St. Jude Medical Inc. announced the launch of the company’s Optis Mobile System in Japan and Europe. The diagnostic system is designed to couple state-of-the-art optical coherence tomography (OCT) and angiography co-registration with fractional flow reserve (FFR) technology into one portable system for hospitals with multiple catheterization labs.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Stentys announced the first distribution agreements for its drug-eluting stent for treating below-the-knee (BTK) arteries in Germany and Belgium, making it the first self-expanding drug-eluting stent commercialized for this indication in Europe.
The widespread belief that radiation from X rays, CT scans and other medical imaging can cause cancer is based on an unproven, decades-old theoretical model, according to a study published in the American Journal of Clinical Oncology.
Contrary to current clinical belief, regular caffeine consumption does not lead to extra heartbeats, which, while common, can lead in rare cases to heart- or stroke-related morbidity and mortality, according to UC San Francisco (UCSF) researchers.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Early diagnosis and treatment of sleep apnea may reduce six-month readmissions for patients hospitalized with heart failure, according to research recently published online by the American Journal of Cardiology.
February 4, 2016 — Stereotaxis and Philips have signed an addendum pursuant to their existing Development and ...
New research from scientists at Sanford Burnham Prebys Medical Discovery Institute (SBP) published in the journal Circulation, may lead to a new approach to help treat heart failure early in the disease.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Biotronik announced CE approval for its new Ilivia implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy devices (CRT-Ds), improving magnetic resonance imaging (MRI) access for cardiac device patients. Ilivia devices come with the company’s ProMRI technology, as well as MRI AutoDetect.
Abbott and Alere Inc. announced a definitive agreement for Abbott to acquire Alere. Under the terms of the agreement, Abbott will pay $56 per common share at a total expected equity value of $5.8 billion.
Medtronic announced Feb. 3 that the U.S. Food and Drug Administration (FDA) revised the CoreValve’s instructions for use ...


 February 09, 2016
February 09, 2016