September 23, 2015 — New research describes a method, tested in rats, that may someday allow healthcare providers to ...
Hansen Medical Inc. announced it will exhibit its Magellan robotic system at the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Scientific Meeting Sept. 26-30 in Lisbon, Portugal. The company will showcase the Magellan 6Fr Robotic Catheter that is used by interventional radiologists in embolization procedures, and will conduct Magellan demonstrations.
The U.S. Department of Health and Human Services’ Office of the National Coordinator for Health Information Technology (ONC), in collaboration with over 35 federal partners, released the updated Federal Health IT Strategic Plan 2015–2020 (Plan) September 21.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

Frost & Sullivan released its list of top medical technologies for 2015, featuring an array of sophisticated inventions in medical devices, diagnostics and imaging. These innovations have given birth to less expensive and flexible healthcare solutions; playing a critical role in managing the increasing frequency of chronic diseases around the globe.

As healthcare converts to an entirely electronic health record (EHR)-based system, it opens up new opportunities to ...
With the world’s elderly population expected to double by 2050, understanding how aging affects the body is an important focus for researchers globally. Cardiovascular disease, the No. 1 cause of death worldwide, often is associated with aging arteries that restrict blood flow. Now, University of Missouri researchers have identified an age-related cause of arterial dysfunction, a finding that could lead to future treatments for some forms of vascular disease.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

The U.S. Food and Drug Administration (FDA) will hold a pair of public workshops in November to explain ongoing efforts to develop regulations and standards for next-generation sequencing (NGS)-based clinical tests. The focus on NGS tests is part of President Obama’s Precision Medicine Initiative (PMI) to help customize healthcare for individuals based on genetic makeup.
University of Texas Southwestern cardiologist Benjamin Levine, M.D., will use NASA-honed technology to monitor swimmer Ben Lecomte in his record-setting goal to become the first person to swim across the Pacific Ocean. Lecomte will plunge into the ocean off of a Tokyo beach this summer heading for San Francisco.
Zoll Medical Corp. announced that it has acquired Tel Aviv, Israel-based Kyma Medical Technologies Ltd., which develops technologies to measure early signs of congestive heart failure.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Cook Medical has received premarket approval from the U.S. Food and Drug Administration (FDA) for its lower-profile Zenith Alpha Thoracic endovascular graft.
For the first time, European Society of Cardiology (ESC) guidelines give the highest degree of recommendation for the radial over the femoral approach for coronary angiography and percutaneous coronary intervention (PCI) in patients with acute coronary syndromes (ACS). The ACS without persistent ST-segment elevation (NSTE-ACS) guidelines, drafted by an international multidisciplinary task force, are published online in European Heart Journal and on the ESC website.
St. Jude Medical Inc. announced European CE Mark approval for the 27mm and 29mm Portico transcatheter aortic valve replacement (TAVR) system. The approval follows previous approvals of the 23mm and 25mm Portico valves, allowing St. Jude Medical to offer physicians an expanded range of fully repositionable, retrievable TAVR valve sizes. The Portico valve accommodates a patient’s native anatomy with diameters ranging from 19 to 27 mm.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
SynCardia Systems initiated a Class I recall of the Freedom Driver Systems used with the company’s temporary Total Artificial Heart (TAH-t) on Aug. 6, 2015. The recall focused on a specific part of the Freedom Driver drive mechanism that the company said may fail and cause the device to stop pumping.
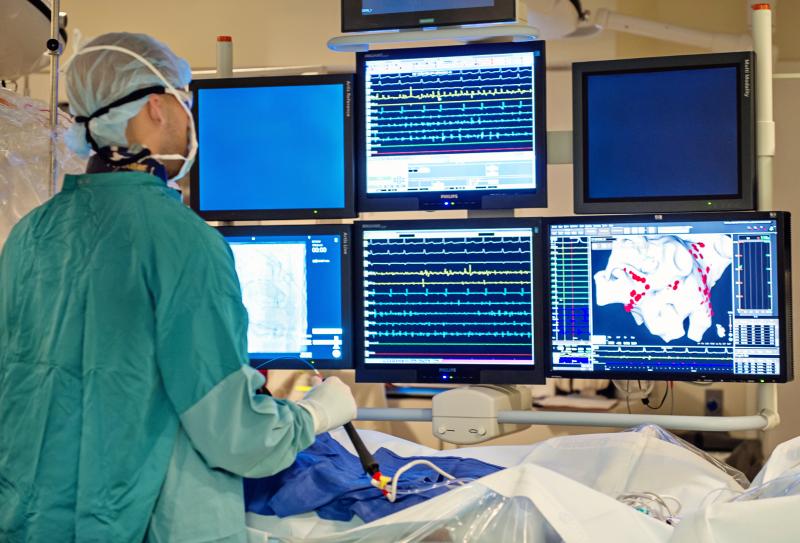
MedLumics has announced the completion of a successful single-case pre-clinical feasibility study of its optical coherence tomography (OCT) technology in the catheter ablation treatment of atrial fibrillation (AF).
At the National Nuclear Security Administration’s (NNSA) 2015 Mo-99 Topical Meeting, Novation offered its support of efforts to encourage the adoption of processes to create a reliable domestic supply of non-highly enriched uranium (HEU) molybdenum-99 (Mo-99).

 September 23, 2015
September 23, 2015