July 25, 2016 — The Centers for Medicare and Medicaid Services (CMS) announced that it expects to launch its new Overall ...
July 26, 2016 — Hematology researchers at The Children’s Hospital of Philadelphia (CHOP) have developed a novel ...
July 26, 2016 — The Centers for Medicare & Medicaid Services (CMS) has proposed the creation of new bundled payment ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

With the recent introduction of several novel oral anticoagulants (NOACs) on the U.S. market that have been billed by ...
Stryker Sustainability Solutions (formerly Ascent Healthcare Solutions) is recalling Angiodynamics Soft Vu Omni Flush Angiographic Catheters due to reports of separation of the tip of the catheter from the main body.
UltraSPECT Inc. announced recently that Robert Wood Johnson Physician Enterprise (RWJPE), a multi-specialty, community-based physician group in Central New Jersey, implemented UltraSPECT’s Xpress3.Cardiac solution at four of their sites.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
July 22, 2016 — The Centers for Medicare & Medicaid Services (CMS) recently announced 516 awardees in 47 states, Puerto ...
CardioKinetix Inc. announced this week that 500 patients have received the company’s Parachute Ventricular Partitioning Device for heart failure. Patients have been treated in more than 15 countries, including patients in key international markets where the device is commercially available, and patients enrolled in PARACHUTE IV, the company’s U.S. pivotal trial under investigational device exemption (IDE).
People who develop heart failure after their first heart attack have a greater risk of developing cancer when compared to first-time heart attack survivors without heart failure, according to a recent study in the Journal of the American College of Cardiology.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
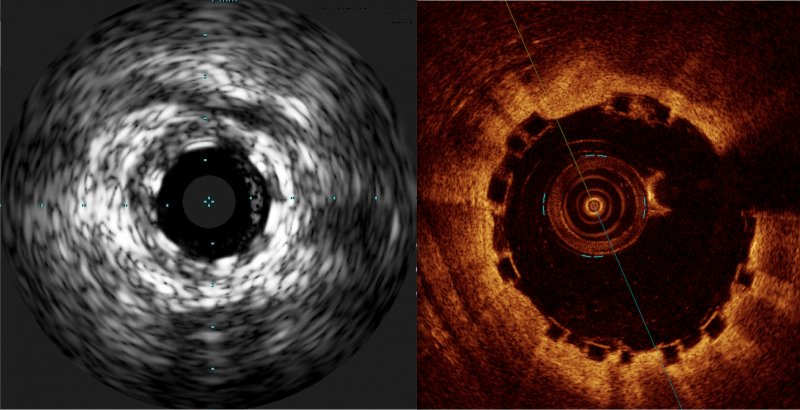
Some have labeled bioresorbable scaffolds (BRS), also known as bioresorbable stents, as the fourth evolution of ...
July 21, 2016 — A new paper published in the June issue of Computer cautions that while mobile health (mHealth) is ...
A novel study has found a simple pre-operative echocardiographic measurement of the amount of torsion of the heart predicted outcomes of mitral valve surgery in some heart failure patients. The study was published in JACC: Basic to Translational Science.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
New data from the TITAN II trial confirm the safety and efficacy of the Carillon Mitral Contour System in the treatment of functional (secondary) mitral regurgitation (FMR).
At the end of June, experts from three different medical societies released a new guideline to help optimize lifetime management of patients with transposition of the great arteries, a congenital heart defect, both before and after surgical intervention.
July 19, 2016 — Siemens Healthineers recently introduced an expanded Services portfolio, known as Enterprise Services ...


 July 26, 2016
July 26, 2016