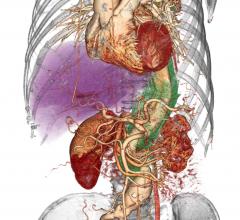
August 30, 2016 — Mission Health and GE Healthcare announced a novel, 10-year outcomes-based agreement that will help ...
Siemens Healthineers announced that the U.S. Food and Drug Administration (FDA) has cleared the Somatom Drive computed tomography (CT) system. This new dual-source scanner is designed to drive precision in diagnostic imaging across a wide range of clinical disciplines, from pediatrics and emergency medicine to cardiology and oncology. The system has the potential to reduce examination time, preparation and follow-up care.
August 29, 2016 — Medtronic plc announced new long-term results from the Medtronic Micra Transcatheter Pacing System ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

August 29, 2016 — Investment research firm Muddy Waters Capital released a report Aug. 25 saying it believes St. Jude ...
This week, PinnacleHealth, Harrisburg, Pa., became the first hospital in the country to implant the Edwards Intuity Elite valve, a rapid deployment device for surgical aortic valve replacement, after U.S. Food and Drug Administration (FDA) approval.
August 26, 2016 — As a further safety measure against the emerging Zika virus outbreak, the U.S. Food and Drug ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
August 25, 2016 — Simulation training for surgery residents builds confidence and could have a life-saving impact on ...
People with a family member who had an aortic dissection — a spontaneous tear in one of the body’s main arteries — should take note of the age that family member was when the aortic dissection occurred. According to a new study published online in The Annals of Thoracic Surgery, aortic dissections have the potential to run in families and often occur within 10 years of the same age.
August 24, 2016 — Whether severe trauma occurs on the battlefield or the highway, saving lives often comes down to ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
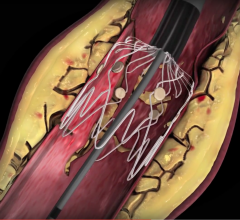
Intact Vascular Inc. announced that the one-year results from its Tack Optimized Balloon Angioplasty (TOBA) clinical study were published in the July 2016 edition of the Journal of Vascular Surgery.
August 24, 2016 — Mayo Clinic has launched a new type of blood test that will be used to predict adverse cardiovascular ...
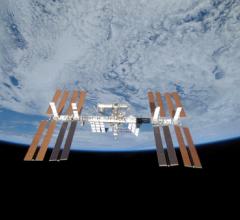
August 24, 2016 — Members of the successful Apollo space program are experiencing higher rates of cardiovascular ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
August 23, 2016 — Technavio analysts forecast the global renal denervation market to grow at a compound annual growth ...
August 23, 2016 — A new study of more than 13,000 people has found that so-called morbid obesity appears to stand alone ...
August 23, 2016 — During a heart attack, clots or narrowed arteries block blood flow, harming or killing cells within ...


 August 30, 2016
August 30, 2016