Medtronic announced the U.S. Food and Drug Administration (FDA) approval and U.S. launch of the CoreValve Evolut R 34 mm valve, now the largest sized transcatheter aortic valve replacement (TAVR) system available in the United States.
Stereotaxis Inc. and Robert Wood Johnson University Hospital (RWJUH) announced that Zyad Younan, M.D., has completed more than 500 cardiac ablation procedures using the Niobe remote magnetic navigation system. Younan currently leads the Northeast region in catheter ablations performed using the Niobe system in the six-and-a-half years since installation.
A recent study from University of Alabama at Birmingham (UAB) researchers published in PLOS ONE compares different available treatments for stroke prevention in patients with non-valvular atrial fibrillation (NVAF).
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The Human Animal Bond Research Initiative (HABRI) announced it has awarded a $44,000 grant to Duke University School of Medicine’s Division of Pediatric Cardiology for a new research study on the impact of therapy dogs on pediatric echocardiograms.

October 25, 2016 — St. Jude Medical is recalling 251,346 of its Fortify, Unify and Assura implantable cardioverter ...
October 24, 2016 — Conventional smart clothing uses conductive fibers or rubber as sensing electrodes, and cardiac ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
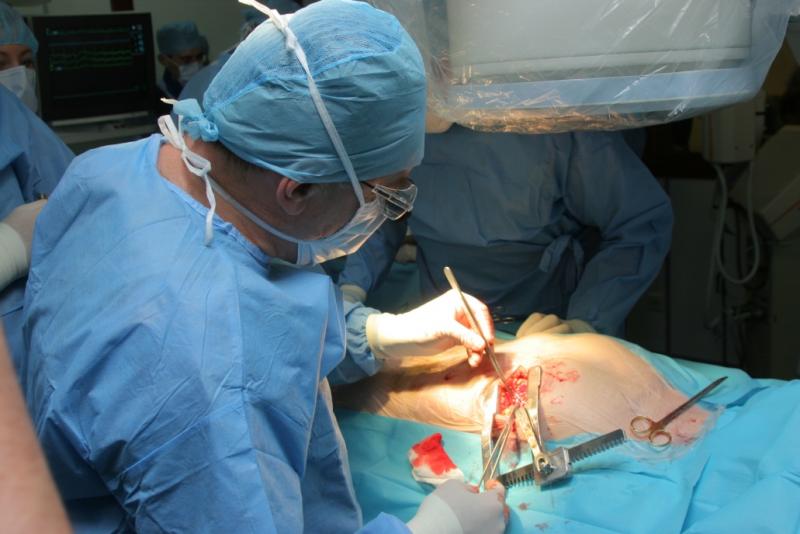
(Editor’s note: This is the second part of a two-part series on the proposed Medicare five-year demonstration for a ...
October 24, 2016 — Medtronic received U.S. Food and Drug Administration (FDA) 510(k) clearance for the HawkOne ...
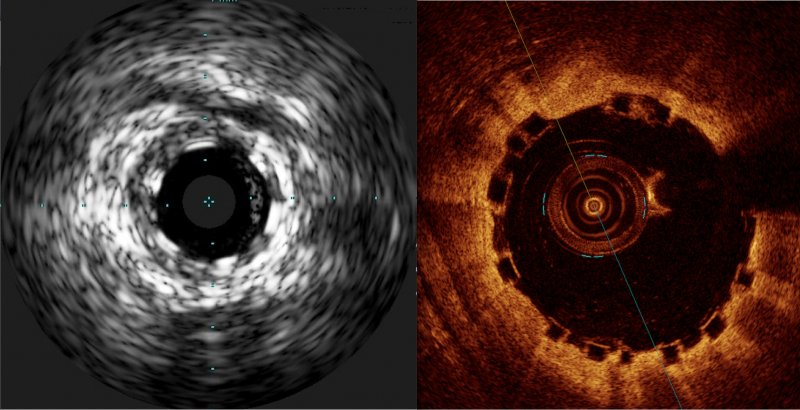
There has been a lot of interest in the interventional community regarding the Abbott Absorb Bioresorbable Vascular ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
October 20, 2016 — SentreHeart Inc. announced in late September the closing of a $35 million Series D round of financing ...
Avinger Inc. recently announced that the company has received expanded indications from the U.S. Food and Drug Administration (FDA) recognizing the Pantheris Lumivascular atherectomy system as a technology that can be used for both therapeutic and diagnostic purposes.
St. Jude Medical Inc. announced the launch of the ADO II AS (AMPLATZER Duct Occluder II Additional Sizes) pediatric clinical trial. The U.S. IDE clinical trial will evaluate the safety and effectiveness of the St. Jude Medical Amplatzer Duct Occluder II AS (ADO II AS), a first-of-its-kind device specifically designed for closure of the small patent ductus arteriosus (PDA).
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Medtronic plc announced last week that it has notified customers of a voluntary recall of certain lots of its Pipeline embolization device, Alligator retrieval device and X-Celerator hydrophilic guidewire. The recall also includes the stylet containing UltraFlow flow directed micro catheters and Marathon flow directed micro catheters. These products are produced, marketed and sold by Medtronic's Neurovascular business, which is part of the Brain Therapies division in the company's Restorative Therapies Group.
As healthcare moves into the era of bundled payments, providers need to be especially focused on ensuring delivery of ...
Analogic Corp. announced last week that it will introduce its new premium cardiac imaging software for the bk3500 ultrasound system at the American College of Emergency Physicians (ACEP) Scientific Assembly, Oct. 16–19, 2016, in Las Vegas.

 October 26, 2016
October 26, 2016














