June 12, 2017 — TandemLife began its commercial launch of the TandemLife Priming Tray, a new product designed to ...
June 12, 2017 — Heart muscle is one of the least renewable tissues in the body, which is one of the reasons that heart ...
June 12, 2017 — A study using strain imaging presented at the American Society of Echocardiography (ASE) 2017 ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
June 12, 2017 – The Medtronic Resolute Onyx Drug-Eluting Stent (DES) met its primary endpoint of target lesion failure ...

June 5, 2017 – The U.S. Food and Drug Administration (FDA) granted market clearance for the Claret Medical Sentinel ...
Chi-Ming Chow, M.D., MSc, FRCPC, FACC, FASE, attending staff cardiologist, St. Michael's Hospital, and associate professor, Department of Medicine, University of Toronto, discusses new technology trends in ultrasound and what developments will effect the future of cardiac ultrasound.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Andrea Natale, M.D., FACC, FHRS, FESC, executive medical director, Texas Cardiac Arrhythmia Institute at St. David's ...

The U.S. Food and Drug Administration (FDA) has granted market clearance for aortic and mitral valve-in-valve procedures using the Edwards Lifesciences Sapien 3 transcatheter heart valve (THV). The Sapien 3 valve is the first transcatheter heart valve approved in the U.S. for the treatment of both aortic and mitral patients who are at high risk for a subsequent open-heart surgery to replace their bioprosthetic valve.
June 1, 2017 — More than 50 companies and organizations will display their latest products and services at the American ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
June 1, 2017 — Here is the list of the most popular pieces of content on the Diagnostic and Interventional Cardiology ...
Northwell Health recently announced a new partnership agreement with Peerbridge Health Inc., to explore the future of remote medical monitoring. Peerbridge technology enables remote patient monitoring through multiple on-body wireless sensors.
June 1, 2017 — Infab Corp. recently introduced Revolution Lead and Lead-Free Aprons with Kiarmor, Infab’s proprietary bi ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Lucas Boersma, M.D., Ph.D., FESC, St. Antonius Ziekenhuis, Nieuwegein, The Netherlands, discusses how subcutaneous ...
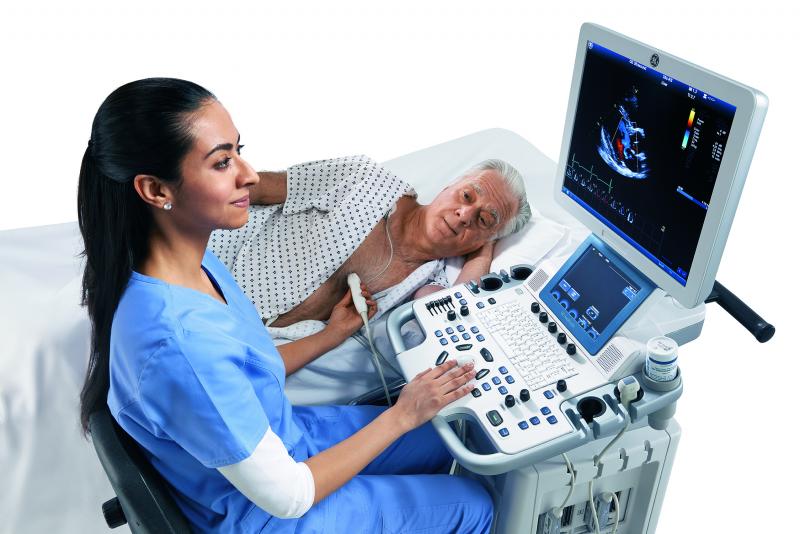
ASE Announces Meeting Highlights for 28th Annual Scientific Sessions — The program includes learning opportunities from ...
LivaNova PLC recently announced the presentation of data from multiple studies demonstrating the safety and effectiveness of the PercevalM sutureless valve for aortic valve replacement (AVR) patients and the Memo 3D ReChord for mitral valve repair. The three data presentations on Perceval, which included a late-breaking clinical trial and a poster presentation on the Memo 3D ReChord, were unveiled at the American Association for Thoracic Surgery (AATS) Centennial meeting in Boston, April 29 – May 3.


 June 12, 2017
June 12, 2017