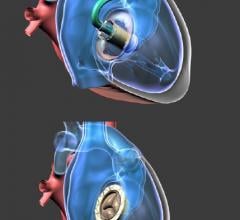
NaviGate Cardiac Structures Inc. (NCSI) announced that its Gate catheter-guided tricuspid atrioventricular valved stent (AVS) was implanted through the jugular vein six weeks ago into a patient’s transplanted heart that was failing due to severe tricuspid valve insufficiency. The successful implantation of the Gate AVS at the Policlinico of the University of Padua, Italy, represents the first European patient treated with the NCSI tricuspid replacement heart valve. Three hours after the intervention the patient was awake and showing improved renal function. Now, approximately two months post-procedure, the patient continues to demonstrate clinical improvement and excellent valvular function. This brings the total number of NCSI Gate tricuspid implants without 30-day mortality to three.
This video educational session, provided in partnership with the American Society of Echocardiography (ASE), is titled ...
This video educational session, provided in partnership with the American Society of Echocardiography (ASE), is titled ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
This video educational session, provided in partnership with the American Society of Echocardiography (ASE), is titled ...
This video educational session, provided in partnership with the American Society of Echocardiography (ASE), is titled ...
Vascular Dynamics Inc. announced interim results of the company’s first-in-human trial of its MobiusHD implant presented in a podium presentation at the European Society of Cardiology (ESC) in Barcelona. The data showed an average reduction of ambulatory systolic blood pressure of 20 mmHg from baseline in the first 40 patients (of an anticipated 50) to reach the six-month endpoint in studies conducted in the U.S. and E.U.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Azeem Latib, M.D., MBBCh, FCP, interventional cardiologist at Columbus Hospital in Milan, Italy, discusses the latest ...
ARTMS Products Inc. signed a strategic partnership with GE Healthcare around ARTMS’ proprietary QUANTM99 Irradiation System (QIS) for radioisotope production. The goal of the partnership is to supply equipment, technologies and research that supports the production and processing of radioisotopes on GE’s PETtrace 800 platform of medical cyclotrons. The QIS integrates on to the GE PETtrace 800 series cyclotron to enable an alternative, non-reactor supply of valuable medical isotopes including technetium-99m, copper-64, gallium-68 and zirconium-89.
Abbott announced it has received U.S. Food and Drug Administration (FDA) approval for its Full MagLev HeartMate 3 Left Ventricular Assist System. The HeartMate 3 system provides a new option for physicians managing advanced heart failure patients in need of short-term hemodynamic support (bridge-to-transplant or bridge to myocardial recovery). The system also provides patients living with their device new benefits that embody the evolution of left ventricular assist device (LVAD) therapy, such as improved blood flow in a pump that uses full magnetic levitation to reduce trauma to blood passing through the system.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

August 29, 2017 — The U.S. Food and Drug Administration (FDA) approved a firmware update that is now available to reduce ...
August 29, 2017 — The European Medicines Agency (EMA) issued a final opinion that recommended restricting the use of ...
C.R. Bard Inc. announced the Lutonix 035 Drug Coated Balloon PTA Catheter (DCB) has been granted premarket approval (PMA) by the U.S. Food and Drug Administration (FDA) for a new indication. With this approval, the device becomes the first and only drug-coated balloon that is FDA-approved as safe and effective in end-stage renal disease (ESRD) patients with stenotic lesions in dialysis arteriovenous (AV) fistulae.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
This video educational session, provided in partnership with the American Society of Echocardiography (ASE), is titled ...
This video educational session, provided in partnership with the American Society of Echocardiography (ASE), is titled ...
August 28, 2017 — Bristol-Myers Squibb Company and Pfizer Inc. announced results from an analysis of real-world data ...

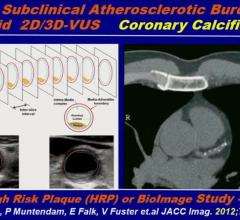
 August 31, 2017
August 31, 2017