Pfizer Inc. announced that the Tafamidis Phase 3 Transthyretin Cardiomyopathy (ATTR-ACT) study met its primary endpoint with a statistically significant reduction in all-cause mortality plus frequency of cardiovascular-related hospitalizations compared to placebo at 30 months. The preliminary safety data showed that tafamidis was generally well tolerated in this population and no new safety signals were identified.1
YMCA of the USA (Y-USA) and the American Heart Association (AHA) are combining efforts to help more people better manage their blood pressure. Beginning this spring, more than 100 clinics participating in the Target: BP initiative will be able to refer qualified patients to enroll at no cost in the YMCA’s Blood Pressure Self-Monitoring program.
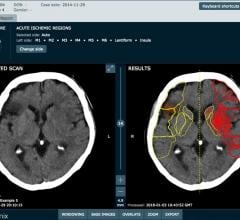
April 5, 2018 — Medical imaging company Brainomix has attracted £7m ($9.8 million) of investment to bring its artificial ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
April 5, 2018 — Diagnostic and Interventional Cardiology (DAIC) magazine received the 2018 Jesse H. Neal Award for Best ...
The Ohio State University Wexner Medical Center will establish the nation’s first center dedicated to treating those with heart failure and arrhythmia with gifts totaling $18 million from Bob and Corrine Frick.
Boston Scientific Corp. announced the acquisition of Securus Medical Group Inc., a privately-held company that has developed a thermal monitoring system for the continuous measurement of esophageal temperature. Boston Scientific has been an investor in Securus since 2016, and the transaction price for the remaining stake not already owned consists of $40 million in cash up-front, as well as up to $10 million in contingent payments based on regulatory achievements and commercial milestones.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
April 4, 2018 — The U.S. Food and Drug Administration (FDA) has cleared the Somatom Edge Plus computed tomography (CT) ...
April 3, 2018 — Following the unprecedented Gore REDUCE Clinical Study conclusion that closure of patent foramen ovale ...
GE Healthcare announced the sale of its Value-Based Care Division — including its software assets for Enterprise Financial Management, Ambulatory Care Management and Workforce Management — to private equity investment firm Veritas Capital. The sale, expected to be completed in third quarter 2018, was valued at $1.05 billion in cash.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

Here is the list of the most popular articles and videos on the Diagnostic and Interventional Cardiology (DAIC) magazine ...
Abiomed Inc. announced that it received U.S. Food and Drug Administration (FDA) Pre-Market Approval (PMA) for its Impella CP heart pump with SmartAssist, utilizing an optical sensor. The company said these advances in technology and software are designed to improve productivity, ease of use and patient management to ensure optimal patient care.
Revised guidelines for the nonsurgical, image-guided interventional treatment of acute ischemic stroke will ensure patients receive the right treatment at the right time. The updated guidelines, endorsed by the Society of Interventional Radiology (SIR) and 11 other medical societies, were published in the April edition of SIR’s peer-reviewed journal, the Journal of Vascular and Interventional Radiology (JVIR).
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Philips in partnership with Innovative Imaging Technologies (IIT) announced an industry-first integrated tele-ultrasound solution on Philips’ Lumify portable ultrasound system powered by IIT’s Reacts collaborative platform. This innovation connects clinicians around the globe in real time by turning a compatible smart device into an integrated tele-ultrasound solution, combining two-way audio-visual calls with live ultrasound streaming.
March 30, 2018 — The Marcus Foundation has donated $15 million to establish the Marcus Stroke Network, a coordinated and ...
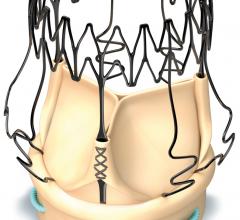
LivaNova PLC announced the first patient enrollment in the Perceval Valve Clinical study for Chinese Registration (“PERFECT”) Trial. The study is a pre-market, prospective, single-arm trial. The PERFECT trial is being conducted to demonstrate the safety and effectiveness of the Perceval sutureless aortic heart valve when used to replace a diseased native or malfunctioning prosthetic aortic valve in the indicated Chinese population for tissue heart valve replacement.


 April 06, 2018
April 06, 2018