Here are the late-breaking trials and other key study presentations from the 2018 EuroPCR conference. This is the annual ...

May 31, 2018 — The results from the Survival Improvement in Extensive Myocardial Infarction with PERsistent Ischemia ...

May 30, 2018 — Despite the best efforts of clinicians to manage hypertension, it continues to increase and is now ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Data from the GENETIC-AF trial was presented in a “Late Breaking Clinical Trials” oral presentation at the European Society of Cardiology (ESC) Heart Failure 2018 World Congress, May 26-29 in Vienna, Austria. William T. Abraham, M.D., professor of medicine, physiology and cell biology and director, Division of Cardiovascular Medicine at the Ohio State University presented the data.
Shockwave Medical recently announced the European commercial availability of Intravascular Lithotripsy (IVL) for calcified coronary artery disease (CAD). The company also announced the first patient enrollment in the DISRUPT CAD II post-market study by Prof. Jonathan Hill, M.D., at King’s College in London.
ReCor Medical announced that the RADIANCE-HTN SOLO study met its primary efficacy endpoint and demonstrated a statistically significant reduction in blood pressure in hypertensive patients. In addition, the company announced submission of an investigational device exemption (IDE) supplement to the U.S. Food and Drug Administration (FDA) for a pivotal study of its Paradise System for the treatment of hypertension. Prof. Ajay Kirtane, M.D., of New York-Presbyterian Hospital/Columbia University has taken the role of co-principal investigator for RADIANCE-HTN.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
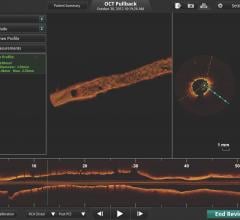
Investigators recently unveiled clinical data from the independently run Onyx 1-Month OCT Study showing strong early vessel healing in its target patient population at one month following implantation of the Resolute Onyx drug-eluting stent (DES). The target population contained a high percentage of patients with complex coronary artery disease.

May 29, 2018 — Results from the innovative SYNTAX III Revolution Trial [1] underline the effectiveness of evolving ...
May 29, 2018 — Ongoing controversy exists regarding the role of percutaneous coronary intervention (PCI) for stable ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
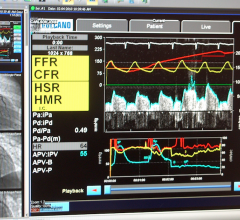
May 29, 2018 — The first placebo-controlled trial that looked at how fractional flow reserve (FFR) and instantaneous ...
M.A. MedAlliance SA has raised $37 million to help develop and commercialize the first sirolimus micro-reservoir drug-coated balloon to treat patients suffering from peripheral artery disease (PAD). The Selution DCB is also intended to treat patients with coronary artery disease (CAD), arteriovenous fistulas (AVF) and grafts (AVG) for end-stage renal disease.
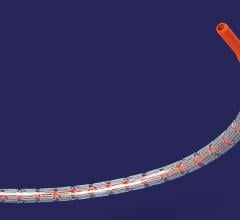
Medtronic plc announced the initiation of a U.S. clinical study to assess the safety and efficacy of drug-eluting stents (DES) for the treatment of bifurcation lesions. Bifurcation lesions account for approximately 20 percent of all percutaneous coronary interventions (PCI).[i] The Bifurcation Cohort, part of the RESOLUTE ONYX Post-Approval Study, will include patients with coronary artery disease (CAD) receiving the Resolute Onyx DES in sizes ranging from 2–5 mm in diameter.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
May 25, 2018 — Data from the first-in-human study using pulsed electric field (PEF) energy ablate heart tissue in the ...
Abbott announced favorable outcomes from the first 100 patients treated in a global study of its Tendyne Transcatheter Mitral Valve Replacement (TMVR) system. According to Abbott, Tendyne is the first and only mitral replacement valve that is repositionable and fully retrievable to allow for more precise implantation, helping improve patient outcomes. The trial is the largest study of a transcatheter mitral valve replacement device to date. Results at 30 days demonstrated that Tendyne is associated with a significant reduction of mitral regurgitation symptoms and low mortality rates.
Abbott announced it received approval from the U.S. Food and Drug Administration (FDA) for Xience Sierra, the newest generation of the company's Xience everolimus-eluting coronary stent system. Design and technology advances in this generation of the device include features specifically designed for the treatment of complex blockages that now account for up to 70 percent of cases.

 May 31, 2018
May 31, 2018