February 8, 2018 — The U.S. Food and Drug Administration (FDA) and the Nuclear Regulatory Commission (NRC) recently took ...
February 26, 2018 – The American Society of Echocardiography (ASE) and its International Alliance Partners are joining ...
February 26, 2018 – Designed specifically for small vessels, Medtronic plc announced U.S. Food and Drug Administration ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
February 23, 2018 — Boston Scientific announced In November 2017 a delay to timelines for commercialization of the Lotus ...
February 23, 2018 — CorFlow Therapeutics AG announced that the company will present new insights into the coronary ...
NaviGate Cardiac Structures Inc. (NCSI)announced that on Feb. 2, 2018, its catheter-guided Gate valved-stent bioprosthesis became the first Canadian orthotopic valve replacement to treat severe tricuspid regurgitation. The procedure was performed at the Quebec Heart and Lung Institute, Laval University (Quebec City, Canada) by the Institute’s Cardiac Team, led by Josep Rodés-Cabau, M.D., interventional cardiologist, an internationally known expert in tricuspid disease therapy; and François Dagenais, M.D., chief of cardiac surgery at the institute. They performed the Health Canada-sanctioned Expanded Access (Compassionate Use) procedure in the patient who presented with severe symptomatic tricuspid regurgitation.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
This video details the first use of a new protocol at The Ohio State University’s Wexner Medical Center to start sudden ...

Interventional cardiology has witnessed a rapid and constant evolution in both techniques and device technology since ...
When someone goes into cardiac arrest and first responders cannot shock their heart back into rhythm, there is virtually no chance of survival. However, a new protocol being tested at The Ohio State University Wexner Medical Center is already saving lives – increasing survival rates from zero to about 40 percent.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
February 22, 2018 — AtriCure Inc. announced that it has launched the AtriClip FLEX•V Left Atrial Appendage (LAA) ...
Vascular closure device provider Cardiva Medical announced that the company has closed on $11 million in additional financing – bringing total equity and debt financing in the current round to $41 million. The additional financing exceeds previous commitments for this round and includes returning equity and debt investors – including PTV Healthcare Capital, Canepa Healthcare, and affiliates of Luther King Capital Management.
The U.S. Food and Drug Administration (FDA) issued the final rule on “Human Subject Protection; Acceptance of Data from Clinical Investigations for Medical Devices.” The rule updates the FDA’s standards for accepting clinical data from clinical investigations conducted both inside and outside the United States to help ensure the protection of human participants, and to help ensure the quality and integrity of data obtained from these clinical investigations.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Corindus Vascular Robotics Inc. announced today that it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for use of its CorPath GRX System in peripheral vascular interventions. The CorPath System is the first and only FDA-cleared medical device, according to the company, to bring robotic precision to both percutaneous coronary intervention (PCI) and peripheral vascular intervention (PVI) procedures.
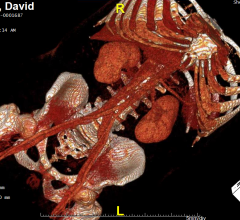
Deep learning startup company Aidoc announced what it calls the world’s first and only comprehensive, full-body solution utilizing artificial intelligence (AI) to help analyze computed tomography (CT) scans, highlighting medical findings for radiologists. The workflow-integrated solution offers support for radiologists covering areas such as the head, c-spine, chest and abdomen.

One of the recent hot topics in interventional cardiology has been how operators can use new tools and techniques to ...

 February 26, 2018
February 26, 2018