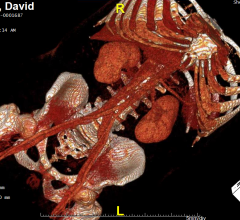
Corindus Vascular Robotics Inc. announced today that it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for use of its CorPath GRX System in peripheral vascular interventions. The CorPath System is the first and only FDA-cleared medical device, according to the company, to bring robotic precision to both percutaneous coronary intervention (PCI) and peripheral vascular intervention (PVI) procedures.
Deep learning startup company Aidoc announced what it calls the world’s first and only comprehensive, full-body solution utilizing artificial intelligence (AI) to help analyze computed tomography (CT) scans, highlighting medical findings for radiologists. The workflow-integrated solution offers support for radiologists covering areas such as the head, c-spine, chest and abdomen.

One of the recent hot topics in interventional cardiology has been how operators can use new tools and techniques to ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
February 20, 2018 – Lexington Biosciences, Inc., a development-stage medical device company, announced the commencement ...
February 20, 2018 – LUMEDX Corporation, a top cardiovascular data intelligence company, will show off the latest in ...
A late-breaking analysis of the landmark COMPASS study was presented at the 2018 International Stroke Conference (ISC), showing that people with chronic coronary artery disease (CAD) and/or peripheral artery disease (PAD) taking Xarelto (rivaroxaban) had fewer ischemic strokes compared to those taking aspirin alone. This analysis, which specifically examined patients from COMPASS who experienced a stroke, also found high-risk patients taking Xarelto plus aspirin had the largest reductions in stroke.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
February 19, 2018 — The Detroit Medical Center’s (DMC) interventional cardiology team at Heart Hospital recently became ...
Claret Medical announced that since U.S. Food and Drug Administration (FDA) clearance in June 2017, its Sentinel Cerebral Protection System has been adopted by 50 U.S. centers as part of its controlled rollout. The company said that some institutions have adopted the device as an emerging standard of care in the U.S. to protect patients from the risk of stroke by capturing and removing debris associated with transcatheter aortic valve replacement (TAVR) before it travels to the brain. The novel system has been shown to significantly reduce the risk of stroke in the first three days after TAVR by more than 60 percent, according to Claret Medical.

For any cardiology department planning to upgrade its cardiovascular picture archiving and communication system (cardiac ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
February 16, 2018 — California’s Petaluma Health Center (PHC) was recently awarded a 2017 Healthcare Information and ...
The father of transradial artery access, Ferdinand Kiemeneij, M.D., Ph.D., interventional cardiologist, The Netherlands ...
DAIC Editor Dave Fornell previews the launch of augmented reality (AR) technology in the March/April 2018 issue of DAIC ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
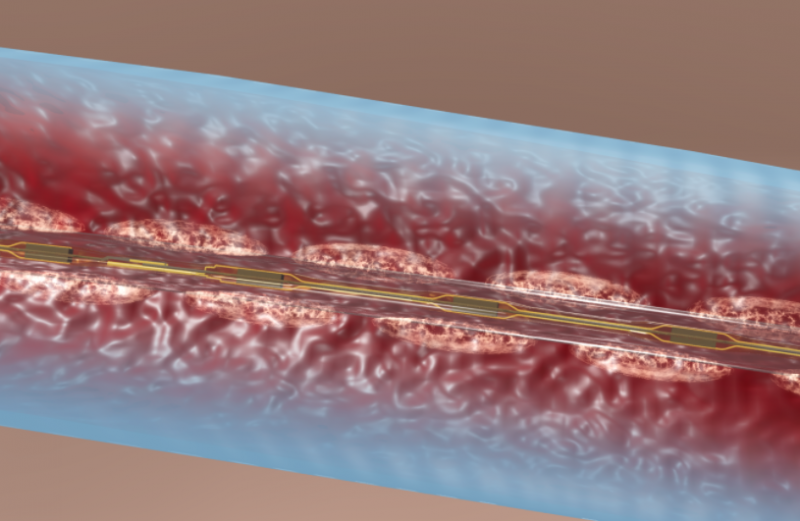
There are few downsides to using tibial venous access to treat deep vein thrombosis (DVT) with catheter-directed ...
The U.S. Food and Drug Administration (FDA) has expanded the indication for Stryker's Trevo Retriever as a front-line treatment for patients experiencing acute ischemic stroke up to 24 hours from symptom onset, increasing the treatment window by 18 hours. The expanded indication of Stryker's clot-removal device is in line with the recently updated treatment guidelines from the American Heart Association (AHA) and American Stroke Association (ASA), and has the potential to reduce disability and improve quality of life for tens of thousands of additional stroke patients each year.
Abiomed Inc. announced that it received an expanded U.S. Food and Drug Administration (FDA) Pre-Market Approval (PMA) for its Impella 2.5, Impella CP, Impella 5.0 and Impella LD heart pumps to provide treatment for heart failure associated with cardiomyopathy leading to cardiogenic shock. This approval expands the previous FDA indication for acute myocardial infarction (AMI) cardiogenic shock and post-cardiotomy cardiogenic shock (PCCS), received in April 2016.


 February 20, 2018
February 20, 2018