Tafamidis is the first treatment to improve survival and reduce hospitalizations in a rare heart condition called transthyretin amyloid cardiomyopathy, according to late-breaking research presented in a Hot Line Session at the European Society of Cardiology (ESC) Congress 2018 and published in the New England Journal of Medicine.
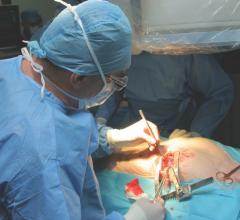
While it is firmly established that the use of one internal thoracic artery can improve life expectancy in coronary artery bypass graft (CABG) surgery compared to vein grafts, it is not known if there are additional benefits with the use of a second artery. This was explored in the ten-year outcomes of the Arterial Revascularisation Trial (ART) presented in a Hot Line Session at the European Society of Cardiology (ESC) Congress 2018, Aug. 25-28 in Munich, Germany.
W. L. Gore & Associates Inc. (Gore) announced the acquisition of Pipeline Medical Technologies Inc., a privately held medical technology company focused on advancing chordal repair for degenerative mitral regurgitation (DMR) via a transcatheter procedure.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The Center for Diagnostic Imaging (CDI) in Miami recently released a list of important preparations patients should make before undergoing a computed tomography (CT) scan.
Transcatheter interventions have become a mainstay in cardiac care as treatments for structural and congenital heart ...
Beyond measuring blood flow, pressure, oxygen levels and other vital signs in the cardiac catheterization lab, current ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Aspirin prevented serious vascular events in patients with diabetes who did not already have cardiovascular disease, but it caused almost as many major bleeds and there was no effect on cancers. These are the late-breaking findings presented in a Hot Line Session at the European Society of Cardiology (ESC) Congress 2018, Aug. 25-28 in Munich, Germany, and published in the New England Journal of Medicine.

The use of invasive, pressure wire-based fractional flow reserve (FFR) in the cath lab is now considered the gold ...
Biotronik announced it is now the exclusive U.S. distributor for the MoMe Kardia external cardiac diagnostic monitor from digital health company InfoBionic. The device benefits patients suspected of experiencing cardiac arrhythmias.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The jury is still out on whether people at moderate risk of a first heart attack or stroke should take daily aspirin to lower their risk, according to late-breaking results from the ARRIVE study. Results from ARRIVE (Aspirin to Reduce Risk of Initial Vascular Events) were presented in a Hot Line Session at the European Society of Cardiology (ESC) Congress 2018, Aug. 25-28 in Munich, Germany, with simultaneous publication in The Lancet.1
Prevencio Inc. announced new data demonstrating its HART PAD test accurately diagnoses peripheral artery disease (PAD) in diabetes mellitus (DM) patients, a patient population in which PAD prevalence has traditionally been difficult to assess. Researchers believe these findings, presented at the 2018 European Society of Cardiology (ESC) Congress, Aug. 25-28 in Munich, Germany, could lead to early identification of PAD and improve patient clinical outcomes, as well as prevent patients without PAD from undergoing unnecessary, expensive and invasive tests.
September 4, 2018 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Philips announced it has acquired Xhale Assurance Inc., a U.S.-based scale-up company developing and commercializing next-generation sensor technologies. Xhale Assurance’s disposable pulse oximetry sensor is placed on the wing (ala) of the nose, and can reliably measure and transmit a patient’s heart rate and blood oxygenation under low-perfusion conditions that are challenging for conventional fingertip pulse oximetry sensors. Philips said this technology will broaden and differentiate its existing portfolio of oxygen saturation monitoring solutions and allow the company to expand into a currently underserved clinical segment. Financial details of the transaction were not disclosed.
A new report from data and analytics company GlobalData suggests that Amazon is poised to make huge strides in healthcare as the Internet retail giant prepares to enter a new market. The report highlights a business model that facilitates easier connectivity between providers and patients, a powerful online platform and its artificial intelligence (AI) interface, Alexa.
A weight loss drug does not increase cardiovascular events, according to late breaking results from the CAMELLIA-TIMI 61 trial. Results were presented in a Hot Line Session at the 2018 European Society of Cardiology (ESC) Congress, Aug. 25-28 in Munich, Germany, and published in the New England Journal of Medicine.


 September 06, 2018
September 06, 2018