The U.S. Food and Drug Administration (FDA) has qualified the Minnesota Living with Heart Failure Questionnaire from the University of Minnesota as part of the Medical Device Development Tools (MDDT) program. This voluntary program is intended to reduce regulatory burden for medical device developers and FDA reviewers by qualifying tools that can aid in the development and evaluation of medical devices. Tools qualified by the FDA can be used by the medical device industry to support device submissions, which could reduce time and resources involved in product development.

The European interventional cardiology market is currently valued at nearly $1.4 billion. This is a mature market that ...
Imran Ahmad, M.D., medical director of interventional cardiology, explains some of the new technologies his labs have ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

In many cases, the diagnosis and management of patients with rare diseases can require the participation of ...

The U.S. Food and Drug Administration (FDA) has approved Portola Pharmaceuticals' Andexxa, the first antidote indicated for patients treated with rivaroxaban (Xarelto) and apixaban (Eliquis), when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding.
Stereotaxis and Acutus Medical announced a strategic collaboration to integrate the Stereotaxis Niobe Magnetic Navigation System and the Acutus Medical AcQMap High Resolution Imaging and Mapping System. The goal of the collaboration is to improve patient care and the physician experience in electrophysiology.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
In March, I made a day trip to New York City to receive the 2017 Jesse H. Neal Award for Best Use of Social Media. The ...
Balancing high quality care with efficiency — and avoiding unnecessary procedures — is a priority for any hospital ...
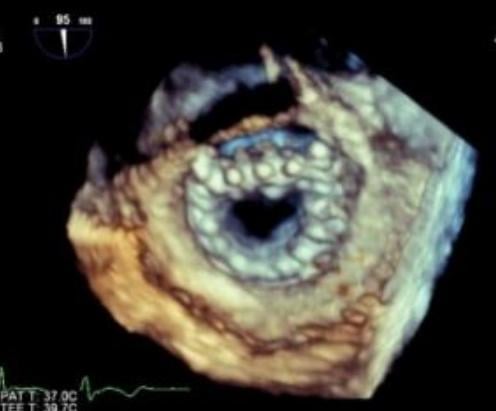
May 4, 2018 — Transcatheter valve technology has been advancing very quickly and the links to aggregated content from ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
May 3, 2018 — The U.S. Food and Drug Administration (FDA) recently announced the availability of a draft guidance for ...
May 3, 2018 — APN Health LLC announced its Navik 3D mapping system is commercially released and in clinical use. The ...
May 3, 2018 — The U.S. Food and Drug Administration (FDA) has granted market clearance for the Abbott Advisor HD Grid ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
May 3, 2018 — The U.S. Food and Drug Administration (FDA) has expanded the indication for the Medtronic In.Pact Admiral ...
May 2, 2018 — Conavi Medical Inc. has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its ...
Select adult patients born with a single functioning ventricle, and who have undergone a surgical operation called the ‘Fontan procedure’ during childhood, are being enrolled in a new global-first clinical trial. The trial, led by a multi-disciplinary team of heart and lung physicians, will examine the effects of a portable, non-invasive medical device never before tested on patients with this cardiac condition.


 May 07, 2018
May 07, 2018












