Endologix Inc. received notice that the U.S. Food and Drug Administration (FDA) has classified a voluntary recall action that Endologix took in July of this year as a Class I recall. The July recall involved Endologix’s issuance of a Safety Notice to healthcare professionals (HCPs) using the AFX Endovascular AAA System.

“I don’t trust anything that’s being portrayed in the marketing brochures and will believe it only when the solution is ...
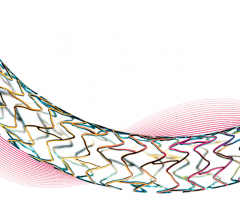
Veryan Medical Ltd has received Premarket Approval (PMA) for the BioMimics 3D Vascular Stent System from the U.S. Food & Drug Administration (FDA). The device is approved for the treatment of symptomatic de novo or restenotic lesions in the native superficial femoral artery and/or proximal popliteal artery.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Teleflex Inc. announced the acquisition of Essential Medical Inc. Based in Exton, Pa., Essential Medical is a privately-held medical device company that has developed and commercialized the CE Marked Manta Vascular Closure Device specifically designed for closure of large bore arteriotomies following procedures utilizing devices or sheaths ranging in size from 10F to 18F (with maximum outer diameters up to 25F).
The Ohio State University Wexner Medical Center is first in the country to test a new medical device designed to help patients with advanced heart failure. Cardiologists here performed the first two implants and the patients went home the next day.
October 4, 2018 — The late-breaking FAST-FFR Trial demonstrated that the sensitivity and specificity of the CathWorks ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
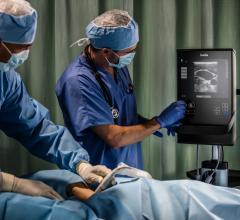
Fujifilm SonoSite Inc. announced its entry into the medical informatics space with the launch of a robust point-of-care workflow solution, SonoSite Synchronicity. The software is designed specifically for point-of-care ultrasound to streamline and standardize clinical workflow while delivering administrative efficiencies with flexible credentialing tools.
October 4, 2018 — Pulmonary artery denervation (PADN) is associated with significant improvements in hemodynamic and ...
Three-dimensional printing company Biomodex, which develops organ twins for advanced physician training, is expanding into the interventional cardiology space with the launch of the Left Atrial Appendage Closure Solution (LAACS). Unveiled at the 2018 Transcatheter Cardiovascular Therapeutics (TCT) conference, Sept. 21-25 in San Diego, the LAACS allows physicians to train on an ultra-realistic, multi-material, 3-D-printed heart.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
October 4, 2018 – Investigators unveiled clinical data from the independent BIONYX and SORT OUT IX all-comers trials ...
DAIC Editor Dave Fornell takes a tour of some of the most innovative new cardiovascular technology he found on the expo ...
Image Diagnostics has entered into a product development agreement with ECLS Inc. to design a new radiation shield expected to debut at the 2018 Radiological Society of North America (RSNA) 2018 annual meeting, Nov. 26-30 in Chicago. The radiation shield, conceptualized by ECLS founder James Goldstein, M.D., cardiologist at Royal Oak Michigan’s Beaumont Hospital, is expected to address the adverse health effects of radiation exposure and the strain of long-term wear of protective lead during interventional surgery.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...

One of the key, early pioneers who helped bring coronary angioplasty to the United States in the late 1970s was Simon ...
A high number of patients in a study who underwent transcatheter aortic valve replacement (TAVR) experienced severe and moderate cases of prosthesis-patient mismatch (PPM), meaning the implanted heart valve is too small for the patient, which can lead to inadequate blood flow. The research team from Penn Medicine also found that the risk of death and of heart failure readmissions were 19 percent and 12 percent higher, respectively, after one year as compared to patients without severe PPM.
Two-year results of the Moderato I Study demonstrated immediate, substantial and sustained reduction in blood pressure when BackBeat cardiac neuromodulation therapy (CNT) was used in patients with persistent hypertension (office BP > 150 mmHg). Patients in the study had persistent hypertension despite two or more anti-hypertensive medications and an indication for a pacemaker.


 October 08, 2018
October 08, 2018