Computed tomography (CT)-based measures of calcification in the abdominal aorta are strong predictors of heart attacks and other adverse cardiovascular events — stronger even than the widely used Framingham risk score. These assertions are according to a new study published in the journal Radiology.
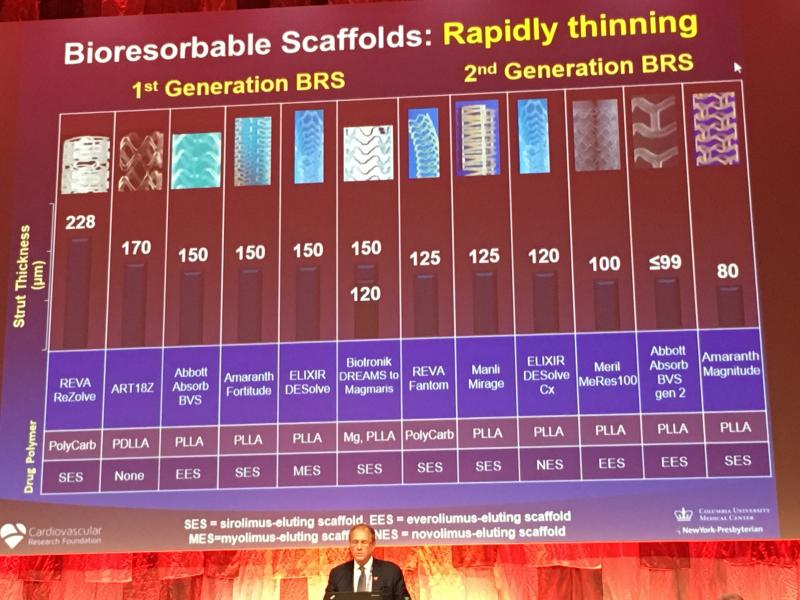
Bioresorbable stent (BRS) technology is not dead, but the unbridled enthusiasm seen two years ago for the technology has ...
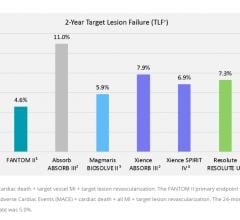
October 12, 2018 – Reva Medical presented four key data sets demonstrating the capabilities of the company’s Fantom ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The Council on Radionuclides and Radiopharmaceuticals Inc. (CORAR) — the voice of the radionuclide, radiopharmaceutical and nuclear pharmacy industries in North America — strongly supports legislation recently introduced by Congressman George Holding (R-NC-2) and Seth Moulton (D-MA-6). This legislation, designated H.R. 6948 - Medicare Diagnostic Radiopharmaceutical Payment Equity Act of 2018, would direct the Secretary of Health and Human Services to recognize diagnostic radiopharmaceuticals (RPs) as drugs and institute a separate payment policy under the Medicare Hospital Outpatient Prospective Payment System (OPPS).
Professor Ian Meredith, MBBS, Ph.D., global chief medical officer and executive vice president, Boston Scientific ...
A discussion with Professor Ian Meredith, MBBS, Ph.D., global chief medical officer and executive vice president, Boston ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Novel results from the landmark EMPA-REG OUTCOME trial suggest that treatment with Jardiance positively impacts life expectancy in adults with type 2 diabetes and established cardiovascular disease. Using actuarial methods, and assuming that the demonstrated beneficial effects of Jardiance remain consistent with long-term use, Jardiance was estimated to extend life expectancy by 1 to 4.5 years on average, depending on age, when compared with placebo. This analysis suggests that treatment with Jardiance could add years of life. The results were published in the journal Circulation.
Canon Medical Systems USA Inc. launched an all-new program designed to help reduce the leading causes of pain and injury for sonographers from work-related musculoskeletal disorders, which afflict up to 90 percent of clinical sonographers. The Healthy Sonographer Program aims to educate sonographers about best practices to prevent positioning themselves in awkward postures – including reaching and twisting – which can result in injuries to the shoulders, neck and wrists and can be a detriment to patient engagement.
October 10, 2018 — Medical coding software provider ZHealth recently unveiled Etch, the first-ever software platform ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

Years ago, I owned a computer that ran a spreadsheet program called Lotus 1-2-3. After about a year, I needed to perform ...
Biosense Webster Inc. recently received approval from the U.S. Food and Drug Administration (FDA) for its Visitag Surpoint External Processing Unit, and enrollment has begun in its post-market approval study.
The U.S. Food and Drug Administration (FDA) has cleared the Magnetom Sola, a 1.5 Tesla magnetic resonance imaging (MRI) scanner from Siemens Healthineers that brings Siemens’ BioMatrix technology to the 1.5T market. This technology addresses patient anatomical and physiological differences, as well as differences in how users set up and conduct MRI exams, to increase productivity and decrease rescans for improved efficiency and patient satisfaction.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Tracking just seven factors of heart attack patients when they are first admitted to the hospital can help flag those most at risk for 30-day readmission, researchers from UT Southwestern found.
The U.S. Food and Drug Administration (FDA) granted market clearance for FibriCheck, a Belgian medical smartphone application for the detection of heart rhythm disorders. The approval makes FibriCheck the first-FDA approved app for heart rhythm disorders by using only an optical signal originating from a non-medical device such as a smartphone.
Endologix Inc. received notice that the U.S. Food and Drug Administration (FDA) has classified a voluntary recall action that Endologix took in July of this year as a Class I recall. The July recall involved Endologix’s issuance of a Safety Notice to healthcare professionals (HCPs) using the AFX Endovascular AAA System.


 October 12, 2018
October 12, 2018