A prospective, multicenter, multinational study examines how fractional flow reserve (FFR) can impact treatment plans and outcomes in patients with stable coronary artery disease (CAD) or acute coronary syndrome (ACS). Results showed more than one-third of the patients’ initial treatment plans changed after FFR. The findings from the global registry were presented as late-breaking clinical research at the Society for Cardiovascular Angiography and Interventions (SCAI) 2019 Scientific Sessions, May 19-22 in Las Vegas.
Akshay Khandelwal, M.D., director of medical operations at the Henry Ford Heart and Vascular Institute, Detroit, and ...
Quantum Genomics announced the enrollment of the first patient in its QUORUM Phase IIb study of its lead clinical candidate, firibastat, in patients with heart failure after acute myocardial infarction (AMI). Firibastat is a first-in-class brain aminopeptidase A inhibitor, being developed for the treatment of resistant hypertension and heart failure.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
June 14, 2019 – A late-breaking study examined the effects of intravascular ultrasound (IVUS) guided drug-eluting stent ...
June 14, 2019 – An expert consensus statement provides recommendations for optimizing the financial operations of the ...
Bardy Diagnostics Inc. announced that its Carnation Ambulatory Monitor (CAM) was recognized with the “Best New Diagnostic Technology” award in the 2019 MedTech Breakthrough Awards Program. MedTech Breakthrough is an independent organization that recognizes the top companies and solutions in the global health and medical technology market. BardyDx earned the distinction for its P-wave centric ambulatory cardiac patch monitoring and arrhythmia detection technology.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Silk Road Medical Inc. announced the presentation of real-world data for the treatment of patients with carotid artery disease at risk for stroke at the Society for Vascular Surgery 2019 Vascular Annual Meeting (VAM), June 12-15 in National Harbor, Md. In a headline presentation, Mahmoud Malas, M.D., of the University of California, San Diego School of Medicine shared updated results for the ongoing TransCarotid Artery Revascularization (TCAR) Surveillance Project.
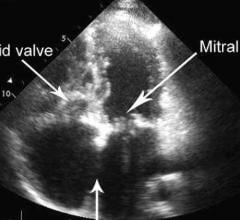
Three-dimensional (3-D) printing software and solutions company Materialise has received U.S. Food and Drug Administration (FDA) clearance for its Mimics Enlight cardiovascular planning software suite. The first release will support clinicians planning complex transcatheter mitral valve replacement (TMVR) procedures.
Orchestra BioMed Inc. announced it has formed a global strategic partnership with Terumo Corp. for development and commercialization of the Virtue Sirolimus-Eluting Balloon (SEB), one of Orchestra’s lead assets, in the percutaneous coronary and peripheral interventions field.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

June 12, 2019 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
Boston Scientific Corp. has initiated the OPTION trial to compare safety and effectiveness of the next-generation Watchman FLX left atrial appendage closure (LAAC) platform to first-line oral anticoagulants (OAC) for stroke risk reduction in patients with non-valvular atrial fibrillation (AF) who undergo a cardiac ablation procedure. OACs used in the trial will include direct oral anticoagulants (DOAC) and warfarin.
Specialized risk scores derived from testing that calculates the cumulative effect of an individual’s entire DNA sequence may reliably predict heart disease in people who have not yet had a heart attack, according to new research. The research is published in Circulation: Genomic and Precision Medicine, an American Heart Association journal.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
In a letter sent to healthcare providers, the U.S. Food and Drug Administration (FDA) validates that Abiomed’s Impella RP heart pump is safe and effective for treatment of right heart failure. The letter comes after the FDA examined the results from Abiomed’s 18-month post-approval study (PAS) of 42 Impella RP patients. The data shows a 64 percent survival rate and 90 percent heart recovery for the subgroup of PAS patients who met the enrollment criteria of Impella RP’s premarket clinical studies. That survival rate is, as the FDA writes in its letter, “similar to the premarket clinical study survival rate,” which was 73 percent.
Centers for Medicare and Medicaid Services (CMS) Administrator Seema Verma addressed the American Medical Association (AMA) Annual Meeting of the House of Delegates, which ran June 8-12 in Chicago. In her remarks, Verma addressed current efforts by the Trump Administration to enact healthcare reform. The following is a transcript of her remarks.
June 11, 2019 — Abbott recently announced positive late-breaking data from its TRILUMINATE study of the company's ...


 June 17, 2019
June 17, 2019