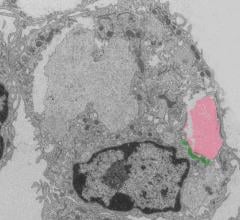
Northwestern Medicine scientists have discovered a novel signaling pathway that promotes healing after a heart attack. The study, published in Cell Metabolism, demonstrates for the first time that the metabolism of dead heart cells by macrophages reprograms the immune cells to launch an anti-inflammatory response and stimulate tissue repair.
Rising temperatures stemming from global climate change may increase the number of infants born with congenital heart defects (CHD) in the U.S. over the next two decades, according to new research in the Journal of the American Heart Association.1 This may also result in as many as 7,000 additional cases over an 11 year-period in eight representative states — Arkansas, Texas, California, Iowa, North Carolina, Georgia, New York and Utah.
January 30, 2019 — Updated atrial fibrillation (AFib) treatment guidelines released this week now recommend new oral ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

In recent years, there has been a lot of focus by vendors on developing better stenting technologies to treat peripheral ...
The U.S. Food and Drug Administration (FDA) has cleared the Magnetom Lumina 3 Tesla (3T) magnetic resonance imaging (MRI) scanner from Siemens Healthineers.
XableCath Inc., announced that its XableCath blunt and abrasion tip catheters were cleared by the U.S. Food and Drug Administration (FDA) as peripheral crossing catheters. The catheters have been safely and successfully used to cross challenging lesions in both arterial chronic total occlusions (CTOs) and chronic obstructive venous lesions. The recent crossing FDA clearance adds to the previous clearances for endovascular support.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
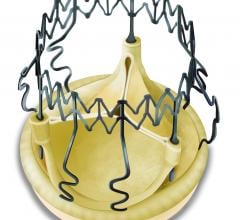
Thubrikar Aortic Valve Inc. announced the first human implant of the Optimum TAV using their transcatheter aortic valve implantation (TAVI) system.
LivaNova PLC announced the publication of three separate studies highlighting the performance of its sutureless aortic valve, Perceval.

There was a lot of hype and high hopes pinned on bioresorbable stent technologies as the way of the future two years ago ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
DAIC Editor Dave Fornell takes a tour of some of the most interesting new artificial intelligence (AI) imaging ...
The American College of Cardiology host the third Advancing the Cardiovascular Care of the Oncology Patient course in Washington on Jan. 25-27, 2019. The event brought together top experts in both cardiology and oncology to review new and relevant science in this rapidly evolving field.
Acutus Medical announced 12-month data from the UNCOVER-AF trial investigating the use of the AcQMap advanced cardiac imaging and mapping visualization system in persistent atrial fibrillation (AF) ablation procedures. Results presented at the 24th Annual AF Symposium, Jan. 24-26 in Boston, show the use of AcQMap resulted in 72.5 percent single-procedure freedom from AF at 12 months. Rigorous post-ablation monitoring showed 89.6 percent of the single-procedure patients experienced zero episodes of AF.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...

There are a few recent trends in X-ray angiography imaging systems that hospitals should be aware of if they are looking ...
Lenox Hill Hospital is the first hospital in New York City to successfully implant Abbott’s Tendyne transcatheter mitral valve replacement (TMVR) system to replace a leaky heart valve. The procedure was performed as part of a national clinical trial testing the safety and efficacy of the device as an alternative to invasive open heart surgery.
Philips announced the latest pooled analysis of patient-level data of over 2,300 patients treated with Philips’ Stellarex Drug-Coated Balloon (DCB) in above-the-knee (ATK) studies, which the company said reinforces the strong safety profile of Stellarex. The independent, third-party pooled analysis demonstrated low mortality rates through three years after the treatment with no device-related deaths.

 January 30, 2019
January 30, 2019