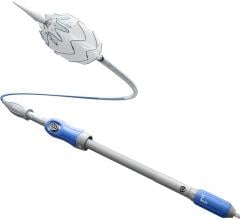
Le Bonheur Children's Hospital cardiologists in Memphis, Tenn., implanted the Amplatzer Piccolo Occluder, the world's first commercially-approved medical device for transcatheter patent ductus arteriosus (PDA) closure in babies weighing as little as two pounds. The self-expanding, wire mesh device developed by Abbott is inserted through a small incision in the leg and guided through vessels to the heart, where it is placed to seal the opening of the heart.
Philips announced the launch of IntelliSpace Cardiovascular 4.1, its next-generation cardiovascular image and information management system. The latest version builds on the existing pediatric reporting capabilities, recognizing the important and unique nature of the pediatric environment. Clinicians can now complete their workflows more efficiently in a web browser, while integration with Philips Forcare enables the sharing of patient data between health systems and hospitals. New features also include enhanced security, enterprise protocols and seamless access to Philips' QLab and Tomtec software tools.
William O'Neill, M.D., highlights best practice protocols based on Impella Quality database and real-world evidence ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Software company Pegasystems Inc. announced new survey data revealing the positive impact of quality patient engagement. Sixty-five (65) percent of consumers agree that communications from their doctor's office motivate them to take action to improve their health, and nearly half responded similarly regarding their primary health insurer. To continue this positive impact, the industry needs to understand how to best engage with individuals for better health outcomes, according to Pegasystems.
A study presented at the 2018 annual meeting of the Cardiovascular and Interventional Radiology Society of Europe (CIRSE) explored the safety and efficacy of the JETI-8 Peripheral Thrombectomy System for the treatment of acute deep vein thrombosis (DVT). The study, presented by Jean Cournoyer-Rodrigue, M.D., CHUM Research Center, Montreal, showed 83 percent technical success.
Information technology (IT) services provider Atos announced the launch of its first comprehensive cybersecurity solution for today's healthcare market. The full offering extends from security consulting services to now including managed security services, cloud security and identity management, increasing security at all levels of patient care.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Heart failure experts at the National Research Center for Cardiac Surgery in Astana, Kazakhstan, recently announced the first successful implantation of the FIVAD (Fully Implanted Ventricular Assist Device) into a human. An article about the implantation was also published in the Journal for Heart and Lung Transplantation.
Over 45 percent of adult atherosclerotic cardiovascular disease (ASCVD) patients suffer financial hardship related to their medical bills, according to a new cardiovascular medicine and society paper. The paper, published in the Journal of the American College of Cardiology, notes that many of these patients cannot pay their medical bills at all.
The American College of Cardiology (ACC), along with nine other cardiology professional societies, recently published a new guidance document for appropriate use criteria (AUC) for multimodality imaging in the assessment of cardiac structure and function in nonvalvular heart disease. The guidance was published online Jan. 7 in the Journal of the American College of Cardiology (JACC).
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
This is an example of an arterial venous malformation (AVM) in the brain imaged on a Canon Alphenix Alpha angiography ...
Medtronic plc recently received U.S. Food and Drug Administration (FDA) approval for the Valiant Navion thoracic stent graft system. The device is indicated for the minimally invasive repair of all lesions of the descending thoracic aorta, including thoracic aortic aneurysms (TAA), blunt thoracic aortic injuries (BTAI), penetrating atherosclerotic ulcers (PAU), intramural hematomas (IMH) and aortic type B dissections (TBAD).
Giving birth is associated with a 14 percent higher risk of heart disease and stroke compared to having no children, reports a study published in the European Journal of Preventive Cardiology. EJPC is a journal of the European Society of Cardiology (ESC).
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Omron Healthcare Inc. and physIQ Inc. announced a collaboration to integrate Omron’s HeartGuide wearable blood pressure monitoring device into the pinpointIQ platform to monitor at-risk patients in an outpatient setting. The strategic collaboration aims to generate actionable clinical information for at-risk patients in an outpatient environment by leveraging the complementary technologies of each company.
Canon Medical Systems USA Inc. recently introduced a new angiography configuration featuring its Alphenix Sky + C-arm and Hybrid Catheterization Tilt/Cradle Table for interventional procedures with its Aquilion One/Genesis Edition computed tomography (CT) system. The new pairing, called the Alphenix 4-D CT, allows clinicians to efficiently plan, treat and verify in a single clinical setting. The flexible hybrid system enables streamlined workflow and extensive range of patient access and coverage.
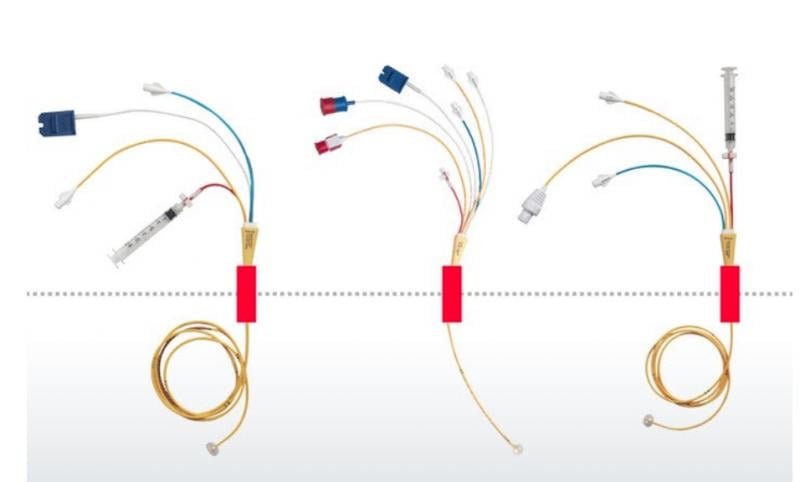
Edwards Lifesciences is recalling its 131F7, 131F7J, 131F7P, 131VF7P, 151F7 Swan-Ganz Thermodilution Catheters ...


 February 13, 2019
February 13, 2019