Expanding its advanced, high-performing Aplio i-series ultrasound platform Canon Medical Systems introduced what it calls the industry’s first 33 MHz ultra-high frequency linear transducer on Aplio i800 to provide extremely fine detail and spatial resolution in the near field.
Robert Klempfner, M.D., director of the Cardiovascular Prevention Institute, Sheba Medical Center, Israel, discusses his ...
The Healthcare Information and Management Systems Society (HIMSS) shared insights on what’s next in health on the heels of the HIMSS19 global conference and exhibition, Feb. 11-15 in Orlando, Fla. This year’s event – which saw 43,000-plus attendees from 90 countries around the world and featured nearly 500 education sessions on 24 education topics – highlighted current industry priorities and offered a glimpse of what the industry can expect to see in the coming year.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Obesity and severe obesity in childhood and adolescence have been added to the list of conditions that put children and teens at increased risk for premature heart disease. The changed was noted in a new scientific statement from the American Heart Association published in the association’s journal Circulation.
How wearable devices will play a role in healthcare was a big topic at the Healthcare Information Management and Systems ...
Canon Medical Systems recently introduced AiCE (Advanced intelligent Clear IQ Engine), a deep convolutional neural network (DCNN) image reconstruction technology for computed tomography (CT). AiCE uses deep learning technology to differentiate signal from noise so that it removes noise while it preserves true signal.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
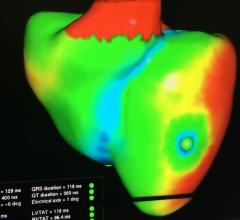
This is a virtual heart with the same electrophysiology characteristics as the real patient being developed to help ...
Ra Medical Systems Inc. announced a 98 percent success rate in the results from a 52-patient study using the company’s DABRA System for the treatment of above- and below-the-knee peripheral artery disease (PAD). The data was presented by Ashok Kondur, M.D., FACC, FSCAI at the 2019 Leipzig Interventional Course (LINC) in Leipzig, Germany.
Philips announced the launch of IntelliSpace Portal 11, the latest release of the company’s comprehensive, advanced visualization and quantification software. Launching at the 2019 European Congress of Radiology (ECR), Feb. 27-March 3 in Vienna, Austria, the new version further extends the clinical innovation of the portal, with enhancements to improve workflow efficiencies, bridging secure data sharing between systems within the hospital network to address the security and privacy needs of customers globally.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Steve Holloway, principal analyst and company director for the healthcare market research firm Signify Research ...
Diagnostic and Interventional Cardiology has been recognized as a finalist in the Jesse H. Neal Awards for the third year in a row. DAIC editor Dave Fornell was named a finalist in the Best Range of Work by a Single Author category for his reporting from the Transcatheter Cardiovascular Therapeutics (TCT) 2018 conference, that included blog posts and video coverage.
GE Healthcare announced Feb. 25 the sale of its biopharmaceutical business to Danaher, and GE Chairman and CEO Larry Culp Jr. said the deal signified that a planned 2019 initial public offering (IPO) for GE Healthcare is now unlikely. GE is reportedly weighing its options following the deal, which casts off the biopharmaceutical business for $21.4 billion.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Here is what I thought were the top five most important take-away presentations from the 2019 Society of Thoracic ...
Philips will unveil a new mixed reality concept developed together with Microsoft that the company says is designed for the operating room of the future. Based on Philips’ Azurion image-guided therapy platform and Microsoft’s HoloLens 2 holographic computing platform, the companies will showcase novel augmented reality applications for image-guided minimally invasive therapies. The technology will debut at Mobile World Congress (MWC) Barcelona, Feb. 25-28 in Barcelona, Spain.

The Society for Cardiovascular Angiography and Interventions (SCAI) 2019 Scientific Sessions in Las Vegas, May 19-22 ...


 March 01, 2019
March 01, 2019