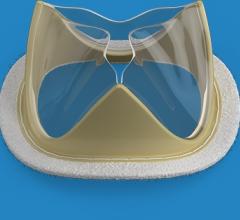
Foldax Inc. announced the U.S. Food and Drug Administration (FDA) has granted investigative device exemption (IDE) approval for an Early Feasibility Study of the Tria surgical aortic heart valve to treat aortic valve disease.
Philips announced the launch of Philips Zenition, its new mobile C-arm imaging platform. Mobile C-arms are X-ray systems that are brought into the operating room (OR) to provide live image guidance during a wide range of surgeries including orthopedic, trauma and vascular procedures. The Zenition mobile C-arm platform brings together innovations in image capture, image processing, ease-of-use and versatility pioneered on Philips’ Azurion platform. Zenition allows hospitals to maximize OR performance, enhance their clinical capabilities and offer their staff a high-quality user experience. Zenition will be introduced in the U.S., Germany, Austria and Switzerland in the first half of 2019, and will subsequently be rolled out in further markets.
Epsilon Imaging recently launched the new EchoInsight Pro Starter configuration for those programs that may be budget constrained or just getting started with the incorporation of strain imaging into echo studies. The EchoInsight Pro Starter configuration starts with a two-seat bundle at an affordable, discounted package price, and can scale to up to six client seats.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
IBM Watson Health and the Broad Institute of MIT and Harvard are launching a research partnership aimed at developing powerful predictive models that will enable clinicians to identify patients at serious risk for cardiovascular disease. This three-year project will incorporate population- and hospital-based biobank data, genomic information, and electronic health records to build upon and expand the predictive power of polygenic scoring. The project will also plan to make insights and tools widely available to the research community, including methods to calculate an individual’s risk of developing common diseases based on millions of variants in the genome.
Corindus Vascular Robotics Inc. is seeking premarket clearance from the U.S. Food and Drug Administration (FDA) to use the CorPath GRX System in neurovascular intervention. CorPath GRX, the second-generation robotic platform, received FDA clearance for percutaneous coronary intervention (PCI) in 2016 and peripheral vascular intervention (PVI) in 2018. Upon successful FDA clearance, CorPath GRX would become the world’s first and only robotic platform indicated for use in PCI, PVI and neurovascular intervention (NVI).
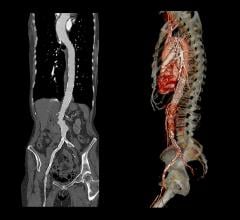
Abdominal aortic stent grafts will remain the largest segment in the global aortic stent graft market at least through 2028 due to the high prevalence of abdominal aortic aneurysms (AAA) relative to thoracic abdominal aneurysms (TAA), according to a new report from GlobalData. The abdominal segment will reach an estimated $2.8 billion global value by 2028, while the overall global aortic stent graft market is expected to reach $4.5 billion in the same period. This represents a compound annual growth rate of 5.4 percent between 2018 and 2028.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Circle Cardiovascular Imaging and Galgo Medical recently announced an agreement for distribution and joint development with respect to magnetic resonance imaging (MRI) scanner application products and workflows, with corresponding post-processing capabilities. The focus will be to improve cardiac MRI workflows and diagnostic tools to expand accessibility and use of the modality to electrophysiology procedures such as atrial fibrillation and ventricular tachycardia ablations.
Medical imaging and visualization company Medivis officially unveiled SurgicalAR, its augmented reality (AR) technology platform for surgical applications, at the 2019 Healthcare Information and Management Systems Society (HIMSS) annual meeting, Feb. 11-15 in Orlando, Fla.
The U.S. Department of Health and Human Services (HHS) has proposed a new rule to support seamless and secure access, exchange and use of electronic health information (EHI).
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
At the 2019 Healthcare Information and Management Systems Society (HIMSS) global conference and exhibition, Feb. 11-15 in Orlando, Fla., Siemens Healthineers will demonstrate how healthcare providers can benefit from digitalization, particularly in the form of artificial intelligence (AI). In addition to its greater digital services portfolio, Siemens Healthineers will showcase two AI-based applications, the AI-Pathway Companion and the AI-Rad Companion.
Le Bonheur Children's Hospital cardiologists in Memphis, Tenn., implanted the Amplatzer Piccolo Occluder, the world's first commercially-approved medical device for transcatheter patent ductus arteriosus (PDA) closure in babies weighing as little as two pounds. The self-expanding, wire mesh device developed by Abbott is inserted through a small incision in the leg and guided through vessels to the heart, where it is placed to seal the opening of the heart.
Philips announced the launch of IntelliSpace Cardiovascular 4.1, its next-generation cardiovascular image and information management system. The latest version builds on the existing pediatric reporting capabilities, recognizing the important and unique nature of the pediatric environment. Clinicians can now complete their workflows more efficiently in a web browser, while integration with Philips Forcare enables the sharing of patient data between health systems and hospitals. New features also include enhanced security, enterprise protocols and seamless access to Philips' QLab and Tomtec software tools.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
William O'Neill, M.D., highlights best practice protocols based on Impella Quality database and real-world evidence ...
Software company Pegasystems Inc. announced new survey data revealing the positive impact of quality patient engagement. Sixty-five (65) percent of consumers agree that communications from their doctor's office motivate them to take action to improve their health, and nearly half responded similarly regarding their primary health insurer. To continue this positive impact, the industry needs to understand how to best engage with individuals for better health outcomes, according to Pegasystems.
A study presented at the 2018 annual meeting of the Cardiovascular and Interventional Radiology Society of Europe (CIRSE) explored the safety and efficacy of the JETI-8 Peripheral Thrombectomy System for the treatment of acute deep vein thrombosis (DVT). The study, presented by Jean Cournoyer-Rodrigue, M.D., CHUM Research Center, Montreal, showed 83 percent technical success.

 February 18, 2019
February 18, 2019