Blood pressure monitoring might one day become as easy as taking a video selfie, according to new research in Circulation: Cardiovascular Imaging, an American Heart Association journal.

There were several interesting new trends in cardiovascular computed tomography (CT) imaging at the 2019 Society of ...
Cardiovascular Systems Inc. (CSI) has acquired the Wirion Embolic Protection System and related assets from Gardia Medical Ltd., a wholly owned Israeli subsidiary of Allium Medical Solutions Ltd.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
When used with a common heart scan, machine learning, a type of artificial intelligence (AI), does better than conventional risk models at predicting heart attacks and other cardiac events, according to a study published in the journal Radiology.
A West Virginia-based rural medical outreach event showcased the use of point-of-care technology in an ambulatory setting and demonstrated that it can enhance clinical decision making. Physicians used new digital health technologies to diagnose acute and chronic cardiovascular diseases in a resource-limited area, and found that it improved their ability to diagnose common cardiac conditions such as atrial fibrillation and heart failure. Research findings on how this technology-based care impacts provider referral and downstream testing were presented during the 30th Annual American Society of Echocardiography (ASE) Scientific Sessions, June 21-25 in Portland, Ore.

New technologies have been developed that may replace the traditional pressure wires and adenosine to assess the ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
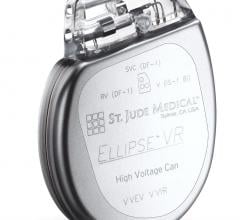
Abbott is recalling the Ellipse Implantable Cardioverter Defibrillators (ICDs) because electrical failures have been identified and determined to be due to a faulty manufacturing process, causing some aluminum wires to be partially exposed. ICDs which contain aluminum wires that are not fully insulated are prone to electrical shorting of the capacitor. The potential patient impact could be the inability to deliver high voltage therapy. There is currently no available method or procedure to determine which of these devices have this issue prior to failure.
The American Society of Radiologic Technologists (ASRT) announced its support for House Resolution (HR) 3772, a measure that loosens certain radiopharmaceutical bundling practices and simplifies Medicare billing for nuclear medicine procedures.
B. Braun Interventional Systems Inc. (BIS) announced the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for the SeQuent Please ReX drug-coated PTCA balloon catheter for the treatment of coronary in-stent restenosis (ISR).
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

Guidewire engineering has become more advanced over the past decade as interventional cardiologists have advanced their trade to tackle much more complex lesions, including chronic total occlusions (CTO). Moving beyond simple, straightforward percutaneous coronary intervention (PCI) lesions to harder-to-reach lesions located in tortuous anatomy, revascularizing completely blocked vessel segments that often require guidewires approaching the CTO both integrate and retrograde and at times using a subintimal approach. This has been the biggest growth area for new guidewires technology.
Here is the list of the most popular cardiovascular news content on the Diagnostic and Interventional Cardiology (DAIC) magazine website from the month of July 2019. This is based on the website’s 261,124 pageviews for the month:
The U.S. Food and Drug Administration (FDA) issued a new draft guidance titled Testing and Labeling Medical Devices for Safety in the Magnetic Resonance (MR) Environment.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Dassault Systèmes announced the five-year extension of its collaboration with the U.S. Food and Drug Administration (FDA). The 3DEXPERIENCE platform will be used to develop a new digital tool to enable more efficient regulatory review of cardiovascular and medical devices. Researchers hope the process will increase industry innovation and pave the way for an efficient path for patients to access safe, effective new treatments for the world’s leading cause of death – heart disease.
Less-invasive procedures to open severely clogged leg arteries were as good at helping people survive and avoid amputation as more invasive open surgeries, according to a new study. The results were reported in Circulation: Cardiovascular Quality and Outcomes, an American Heart Association journal.
Clinical trials are a critical tool for getting new treatments to people who need them, but research shows that difficulty finding the right volunteer subjects can undermine the effectiveness of these studies. Researchers at Cincinnati Children’s Hospital Medical Center designed and tested a new computerized solution that used artificial intelligence (AI) to effectively identify eligible subjects from electronic health records (EHRs), allowing busy clinical staff to focus their limited time on evaluating the highest quality candidates.


 August 06, 2019
August 06, 2019