The U.S. Department of Health and Human Services (HHS) is extending the public comment period by 30 days for two proposed regulations aimed at promoting the interoperability of health information technology (health IT) and enabling patients to electronically access their health information. The new deadline for the submission of comments – June 3, 2019 – will allow additional time for the public to review the proposed regulations.
Researchers at the Icahn School of Medicine at Mount Sinai have demonstrated that the recently developed antidiabetic drug empagliflozin can treat and reverse the progression of heart failure in non-diabetic animal models. Their study also shows that this drug can make the heart produce more energy and function more efficiently. The results were published in the April 23 issue of the Journal of the American College of Cardiology.
A recent study found patients with atherosclerotic cardiovascular disease cut their risk of a second major adverse cardiovascular event by almost 50 percent, if they adhere to taking a statin medication as prescribed by their doctors.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Biotronik announced the European market release of what it calls the world’s smallest implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy defibrillator (CRT-D) devices that are approved for 3T full-body magnetic resonance imaging (MRI) scans.
April 18, 2019 — The U.S. Food and Drug Administration published a draft guidance titled, “Technical Considerations for ...
With Intellispace Enterprise Edition as the foundation, Philips Healthcare is connecting facilities and service areas ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Paul Chang, M.D., professor of radiology, vice chair of radiology informatics and medical director for enterprise ...
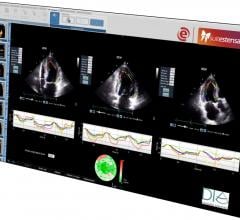
DiA Imaging Analysis has partnered with the Italian healthcare IT company Ebit (Esaote Group), to offer DiA’s LVivo Cardiac Toolbox as an integrated part of Ebit's Suitestensa CVIS (cardiovascular information system). The LVivo Cardiac Toolbox is designed to analyze cardiac ultrasound images based on more objective and reproducible information, as opposed to manual measurement or visual analysis methods that are currently being used.
HonorHealth Research Institute announced the first patients have been enrolled in the SynIVUS-DAPT Study. The clinical study is designed to test outcomes of decreasing the amount of time patients with a high risk of bleeding take antiplatelet medications after receiving a drug-eluting coronary stent.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
CardioFocus Inc. announced the European CE Mark approval of the HeartLight X3 Endoscopic Ablation System.

Over the last 40 years, despite multiple advancements in percutaneous coronary interventions, calcified lesions remain a ...
For the first time in the United States, doctors with the American Heart Association (AHA) have outlined best practices for cardiologists to evaluate and manage patients who have heart attacks with no significant signs of coronary artery disease — a condition known as myocardial infarction with non-obstructive coronary arteries (MINOCA). The new document, published in the March 27 issue of Circulation,1 aims to help physicians better recognize patients with this condition, to avoid common misdiagnoses and streamline care. It is especially important for women, who represent a disproportionate number of MINOCA cases.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...

As we strive to process today’s successive news cycles involving negative reports about immigration, it is easy for many ...
April 15, 2019 – Intact Vascular Inc. received U.S. Food and Drug Administration (FDA) market clearance for the Tack ...
April 15, 2019 — Biotronik began its U.S. commercial launch of the PK Papyrus covered coronary stent system for use in ...


 April 19, 2019
April 19, 2019