Medtronic has received U.S. Food and Drug Administration (FDA) approval for the Attain Stability Quad MRI SureScan left heart lead. Paired with Medtronic quadripolar cardiac resynchronization therapy-defibrillators (CRT-D) and -pacemakers (CRT-P), the Attain Stability Quad lead is the only active-fixation left heart lead, according to the company, and is designed for precise lead placement and stability. The lead will be commercially available in the U.S. in summer 2019.

As the National Association of County and City Health Officials state, healthcare breaches remained to be costly and ...
Abbott announced the launch of a new, smarter heart monitor for better arrhythmia detection — positive news for people at risk for irregular heartbeats. Now with CE Mark in Europe and U.S. Food and Drug Administration (FDA) clearance, the next-generation Confirm Rx insertable cardiac monitor (ICM), a paperclip-sized implantable device, combines smartphone connectivity and continuous, remote monitoring to track unpredictable heart rhythm problems for fast and accurate diagnosis.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
May 6, 2019 — The U.S Food and Drug Administration (FDA) has cleared the Boston Scientific Vici Venous Stent System for ...
May 6, 2019 — The U.S. Food and Drug Administration (FDA) approved tafamidis meglumine (Vyndaqel) and tafamidis ...
Bioengineers have cleared a major hurdle on the path to 3-D printing replacement organs with a breakthrough technique for bioprinting tissues. The new innovation allows scientists to create exquisitely entangled vascular networks that mimic the body's natural passageways for blood, air, lymph and other vital fluids.
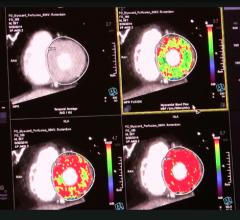
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
A discussion with Gianluca Pontone, M.D., Ph.D., FSCCT, director of cardiovascular MRI, Centro Cardiologico Manzino ...

One of the most promising areas for innovation in healthcare is to be found in the workforce – both in hiring and ...
Coronary artery bypass grafting (CABG) surgery may be the best treatment option for most patients with more than one blocked heart artery, according to research published online in The Annals of Thoracic Surgery.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Technology has made its way into the healthcare sector and brought a drastic transformation. Detection of heart ...
A discussion with Khaldoon Alaswad, M.D., director, cardiac catheterization lab, Henry Ford Hospital in Detroit on ...
Concept Medical Inc. (CMI) has been granted Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) for MagicTouch, its sirolimus drug-coated balloon (DCB) catheter, for the treatment of coronary in-stent restenosis (ISR).
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Medtronic announced the company has received U.S. Food and Drug Administration (FDA) approval for the CareLink SmartSync Device Manager. With the introduction of SmartSync, physicians will now be able to use an Apple iPad to program and manage data from Medtronic's BlueSync-enabled implanted cardiac devices.
Abiomed announced that, on April 26, the U.S. Food and Drug Administration (FDA) approved initiation of the ST-Elevation Myocardial Infarction Door-to-Unloading (STEMI DTU) Pivotal Randomized Controlled Trial. The prospective, multi-center, two-arm trial plans to enroll 668 patients undergoing treatment for a STEMI heart attack. Half the patients will be randomized to receive delayed reperfusion after 30 minutes of left ventricular unloading with the Impella CP. The other half will receive immediate reperfusion, the current standard of care.
This podcast is a discussion with William O'Neill, M.D., director of the structural heart program, Henry Ford Hospital ...


 May 06, 2019
May 06, 2019