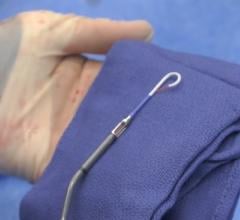
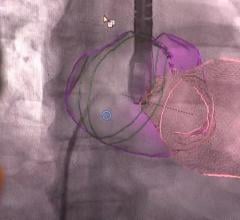
Concept Medical Inc. (CMI) has been granted Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) for MagicTouch, its sirolimus drug-coated balloon (DCB) catheter, for the treatment of coronary in-stent restenosis (ISR).
Medtronic announced the company has received U.S. Food and Drug Administration (FDA) approval for the CareLink SmartSync Device Manager. With the introduction of SmartSync, physicians will now be able to use an Apple iPad to program and manage data from Medtronic's BlueSync-enabled implanted cardiac devices.
Abiomed announced that, on April 26, the U.S. Food and Drug Administration (FDA) approved initiation of the ST-Elevation Myocardial Infarction Door-to-Unloading (STEMI DTU) Pivotal Randomized Controlled Trial. The prospective, multi-center, two-arm trial plans to enroll 668 patients undergoing treatment for a STEMI heart attack. Half the patients will be randomized to receive delayed reperfusion after 30 minutes of left ventricular unloading with the Impella CP. The other half will receive immediate reperfusion, the current standard of care.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
This podcast is a discussion with William O'Neill, M.D., director of the structural heart program, Henry Ford Hospital ...

When Andreas Grüntzig, father of percutaneous transluminal coronary angioplasty, approached his cardiac surgeon ...
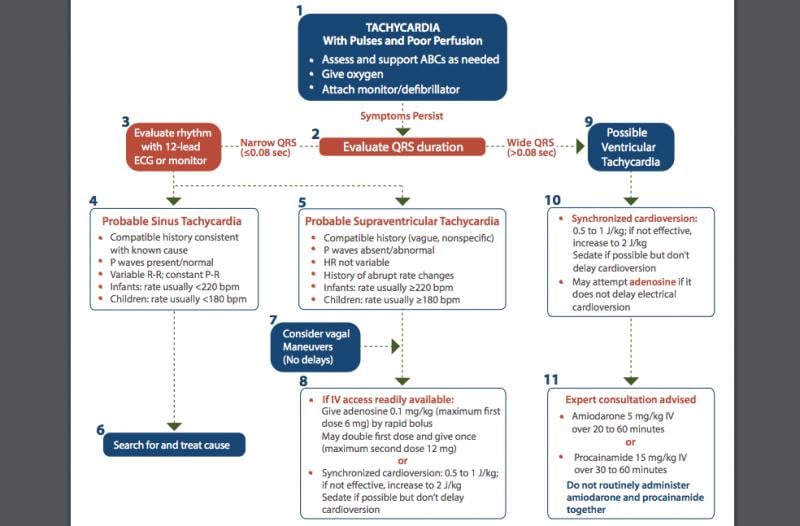
The treatment of all patients in distress with significant symptoms begins with the basics, and pediatric tachycardia is ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

May 1, 2019 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
Alex Haak, Ph.D., clinical scientist at Philips Health Systems North America, is based at the University of Colorado ...
Digital healthcare company Murj announced the immediate availability of the Murj 2.0 implantable cardiac device management software.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
PhaseBio Pharmaceuticals Inc. announced the U.S. Food and Drug Administration (FDA) has granted Breakthrough Therapy designation for PB2452, a novel reversal agent for the antiplatelet drug ticagrelor.
A new document compiled by four cardiac imaging professional societies provides a resource to guide clinicians in best practices for assessing valvular regurgitation following catheter-based repair or replacement of a valve. The document, available in full here, supplements the previously published guideline Recommendations for Evaluation of Prosthetic Valves with Echocardiography and Doppler Ultrasound.
New medical technology offers the promise of improving patient care, as well as the potential for harm if caregivers are not sufficiently educated about its operation and use. Without a comprehensive approach, caregivers may learn how to use complex technology at the patient’s bedside, through trial and error, instruction manuals or informally from their colleagues. An article in AACN Advanced Critical Care describes how Memorial Hermann Health System in Houston developed an interdisciplinary fail-safe process to analyze and scale training for use of medical devices, with a risk assessment tool to predict the severity and frequency of potential harm to patients.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...

The heart and kidneys are inextricably linked through a diverse web of hemodynamic, neural and hormonal mechanisms. As ...
Biotronik announced the full commercial launch of the Acticor device family, including Acticor DX and CRT-DX devices. Electrophysiologists throughout the United States are now treating patients with the new implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy defibrillators (CRT-Ds).
Edwards Lifesciences is recalling the Miller Balloon Atrioseptostomy Catheter and Fogarty Dilation Atrioseptostomy Catheter because of the possibility of difficulty in balloon deflation after deployment, which may lead to balloon fragmentation or detachment upon attempted retrieval. The firm has received reports of balloon fragmentation or detachment, which may cause serious adverse health consequences including: damage to the heart, the inferior vena cava, and/or the femoral and iliac veins; additional procedures to retrieve the fragments; permanent patient disability; pulmonary embolism; stroke; damage to other organs; or death. One serious injury was reported in which an infant underwent an invasive, but successful surgical procedure to retrieve a detached balloon. There were no deaths reported.


 May 02, 2019
May 02, 2019