May 6, 2019 — The U.S. Food and Drug Administration (FDA) approved tafamidis meglumine (Vyndaqel) and tafamidis ...
Bioengineers have cleared a major hurdle on the path to 3-D printing replacement organs with a breakthrough technique for bioprinting tissues. The new innovation allows scientists to create exquisitely entangled vascular networks that mimic the body's natural passageways for blood, air, lymph and other vital fluids.
A discussion with Gianluca Pontone, M.D., Ph.D., FSCCT, director of cardiovascular MRI, Centro Cardiologico Manzino ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

One of the most promising areas for innovation in healthcare is to be found in the workforce – both in hiring and ...
Coronary artery bypass grafting (CABG) surgery may be the best treatment option for most patients with more than one blocked heart artery, according to research published online in The Annals of Thoracic Surgery.
Technology has made its way into the healthcare sector and brought a drastic transformation. Detection of heart ...
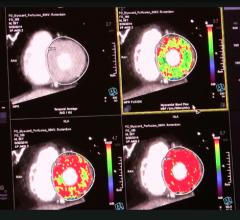
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
A discussion with Khaldoon Alaswad, M.D., director, cardiac catheterization lab, Henry Ford Hospital in Detroit on ...
Concept Medical Inc. (CMI) has been granted Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) for MagicTouch, its sirolimus drug-coated balloon (DCB) catheter, for the treatment of coronary in-stent restenosis (ISR).
Medtronic announced the company has received U.S. Food and Drug Administration (FDA) approval for the CareLink SmartSync Device Manager. With the introduction of SmartSync, physicians will now be able to use an Apple iPad to program and manage data from Medtronic's BlueSync-enabled implanted cardiac devices.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Abiomed announced that, on April 26, the U.S. Food and Drug Administration (FDA) approved initiation of the ST-Elevation Myocardial Infarction Door-to-Unloading (STEMI DTU) Pivotal Randomized Controlled Trial. The prospective, multi-center, two-arm trial plans to enroll 668 patients undergoing treatment for a STEMI heart attack. Half the patients will be randomized to receive delayed reperfusion after 30 minutes of left ventricular unloading with the Impella CP. The other half will receive immediate reperfusion, the current standard of care.
This podcast is a discussion with William O'Neill, M.D., director of the structural heart program, Henry Ford Hospital ...

When Andreas Grüntzig, father of percutaneous transluminal coronary angioplasty, approached his cardiac surgeon ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
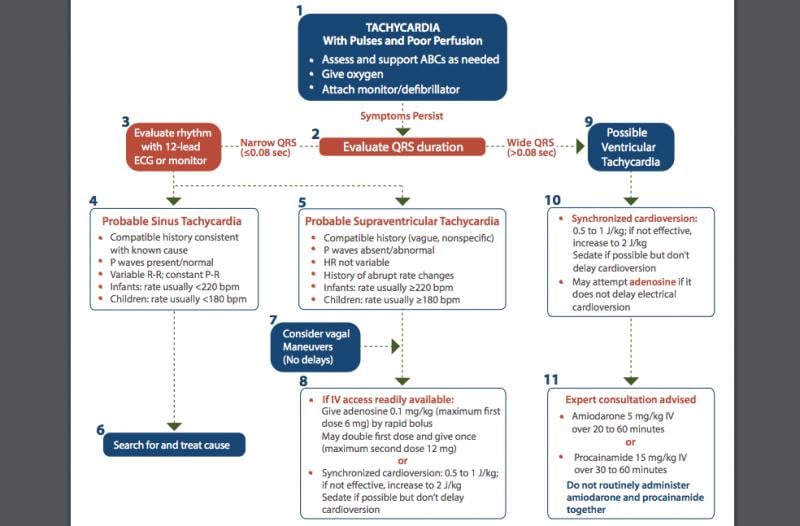
The treatment of all patients in distress with significant symptoms begins with the basics, and pediatric tachycardia is ...

May 1, 2019 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
Alex Haak, Ph.D., clinical scientist at Philips Health Systems North America, is based at the University of Colorado ...


 May 06, 2019
May 06, 2019