Spacelabs Healthcare debuted Sentinel 11 at the 40th Annual Heart Rhythm Society (HRS) Scientific Sessions, May 8-11, 2019, in San Francisco. This latest release from Spacelabs advances cardiology information management with optimized workflows, expanded connectivity and robust data security capabilities.

May 10, 2019 — Here is the list of the late-breaking hot line studies being presented at the 2019 EuroPCR interventional ...
May 10, 2019 — Diagnostic and Interventional Cardiology (DAIC) earned a Bronze Award at the 2019 National Azbee Awards ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
May 10, 2019 — Radiation oncology vendor Varian announced it acquired the start-up company CyberHeart, which has ...
Osprey Medical announced the launch of DyeMINISH, a global patient registry to evaluate the ongoing safety and performance of the DyeVert Contrast Reduction System during standard clinical use in a real-world patient population. The DyeMINISH registry is a retrospective, large-scale, multi-center study, with the first patient included on May 7, 2019. The registry is designed to enroll up to 10,000 participants and is expected to complete in late 2023.
Stereotaxis introduced Stereotaxis Genesis RMN, its next-generation robotic platform that it claims is a significant advancement in robotic magnetic navigation technology.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Philips announced a collaboration with Medtronic to further advance treatment of paroxysmal atrial fibrillation (PAF), a common heart rhythm disorder. Through the agreement, Medtronic will facilitate sales of products on behalf of Philips to provide an integrated image guidance solution for cryoablation procedures. Philips will bring to market the novel KODEX-EPD cardiac imaging and navigation system with cryoablation specific features to enable electrophysiologists to perform cryoablation procedures with reduced need for X-ray imaging.
Even though many medical practitioners may opt not to perform procedures on higher-risk patients, new research finds it may be a good idea for those who suffer from both atrial fibrillation and heart disease.
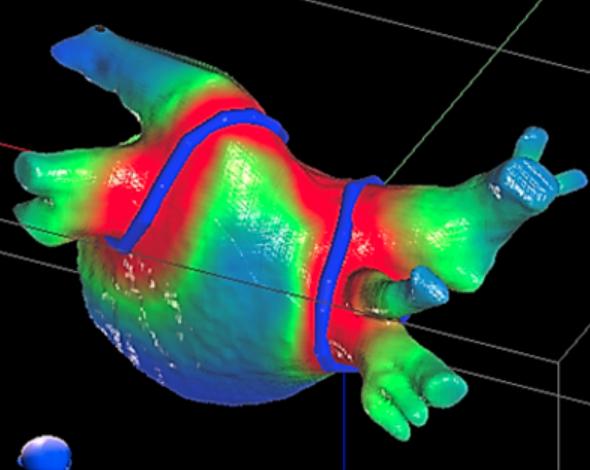
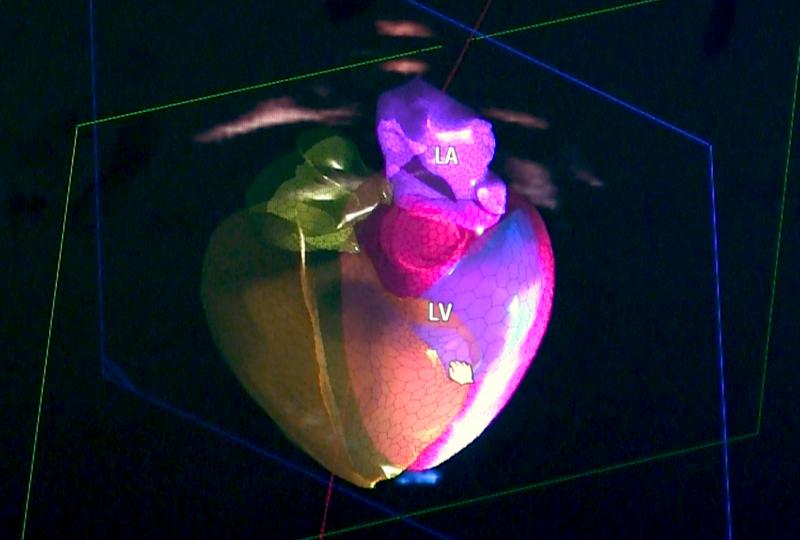
This 360 degree photo shows Dee Dee Wang, M.D., director of structural heart imaging, Henry Ford Hospital, Detroit, Mich ...
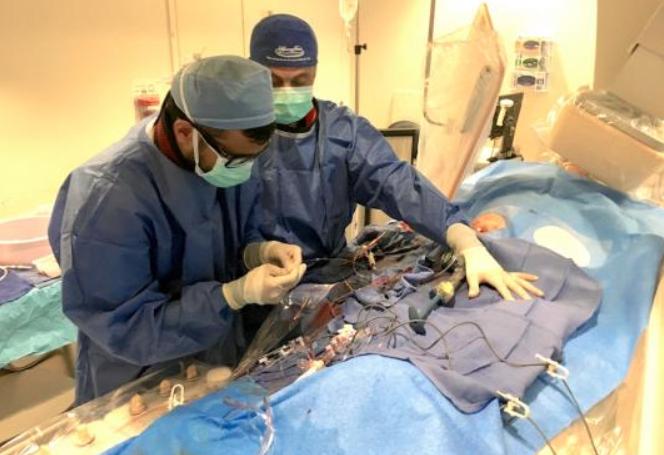
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
May 8, 2019 — The Society for Cardiovascular Angiography and Interventions (SCAI) 2019 Scientific Sessions will take ...
Transcatheter aortic valve replacement (TAVR) has been studied as an alternative to surgical aortic valve replacement in high and intermediate-risk surgical patients for more than five years. A new clinical trial, one of two studies on the topic presented at the American Association for Thoracic Surgery’s 99th Annual Meeting, May 4-7 in Toronto, finds the procedure to be equivalent or potentially preferable to surgical aortic valve replacement (SAVR) for low-risk patients.
The U.S. Food and Drug Administration (FDA) issued a safety communication to alert healthcare providers and patients about issues that may cause batteries in certain Medtronic implantable pacemakers or cardiac resynchronization therapy pacemakers (CRT-Ps) to drain more quickly than expected without warning.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Artificial Intelligence has a multitude of impacts on our daily lives, from recommending movies based upon your Netflix ...
John Carroll, M.D., FACC, FSCAI, director of interventional cardiology and co-director of the Cardiac and Vascular ...
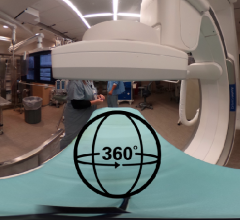
This is a 360 degree image showing the main hybrid cath lab at Henry Ford Hospital in Detroit, which is used for most of ...


 May 10, 2019
May 10, 2019