The U.S. Food and Drug Administration (FDA) issued a safety communication to alert healthcare providers and patients about issues that may cause batteries in certain Medtronic implantable pacemakers or cardiac resynchronization therapy pacemakers (CRT-Ps) to drain more quickly than expected without warning.
Artificial Intelligence has a multitude of impacts on our daily lives, from recommending movies based upon your Netflix ...
John Carroll, M.D., FACC, FSCAI, director of interventional cardiology and co-director of the Cardiac and Vascular ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
This is a 360 degree image showing the main hybrid cath lab at Henry Ford Hospital in Detroit, which is used for most of ...
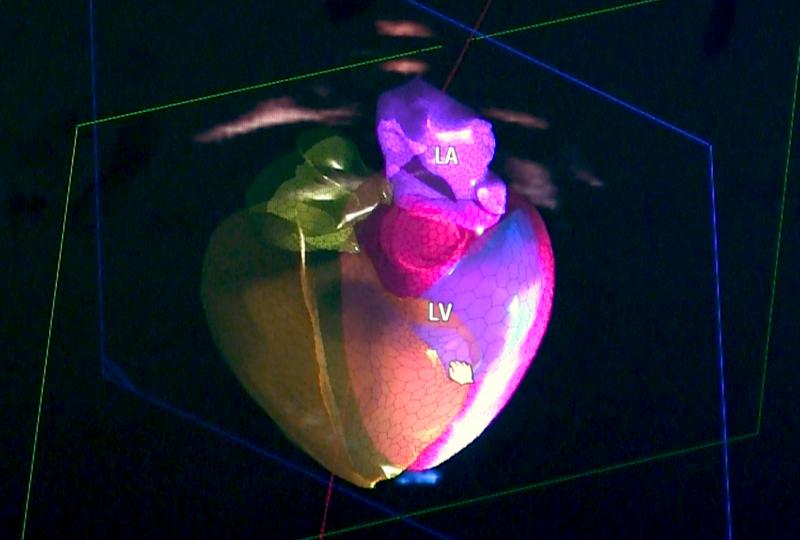
Medtronic has received U.S. Food and Drug Administration (FDA) approval for the Attain Stability Quad MRI SureScan left heart lead. Paired with Medtronic quadripolar cardiac resynchronization therapy-defibrillators (CRT-D) and -pacemakers (CRT-P), the Attain Stability Quad lead is the only active-fixation left heart lead, according to the company, and is designed for precise lead placement and stability. The lead will be commercially available in the U.S. in summer 2019.

As the National Association of County and City Health Officials state, healthcare breaches remained to be costly and ...
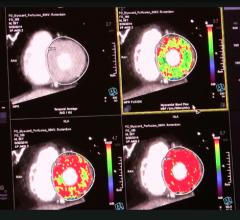
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Abbott announced the launch of a new, smarter heart monitor for better arrhythmia detection — positive news for people at risk for irregular heartbeats. Now with CE Mark in Europe and U.S. Food and Drug Administration (FDA) clearance, the next-generation Confirm Rx insertable cardiac monitor (ICM), a paperclip-sized implantable device, combines smartphone connectivity and continuous, remote monitoring to track unpredictable heart rhythm problems for fast and accurate diagnosis.
May 6, 2019 — The U.S Food and Drug Administration (FDA) has cleared the Boston Scientific Vici Venous Stent System for ...
May 6, 2019 — The U.S. Food and Drug Administration (FDA) approved tafamidis meglumine (Vyndaqel) and tafamidis ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Bioengineers have cleared a major hurdle on the path to 3-D printing replacement organs with a breakthrough technique for bioprinting tissues. The new innovation allows scientists to create exquisitely entangled vascular networks that mimic the body's natural passageways for blood, air, lymph and other vital fluids.
A discussion with Gianluca Pontone, M.D., Ph.D., FSCCT, director of cardiovascular MRI, Centro Cardiologico Manzino ...

One of the most promising areas for innovation in healthcare is to be found in the workforce – both in hiring and ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Coronary artery bypass grafting (CABG) surgery may be the best treatment option for most patients with more than one blocked heart artery, according to research published online in The Annals of Thoracic Surgery.
Technology has made its way into the healthcare sector and brought a drastic transformation. Detection of heart ...
A discussion with Khaldoon Alaswad, M.D., director, cardiac catheterization lab, Henry Ford Hospital in Detroit on ...


 May 07, 2019
May 07, 2019