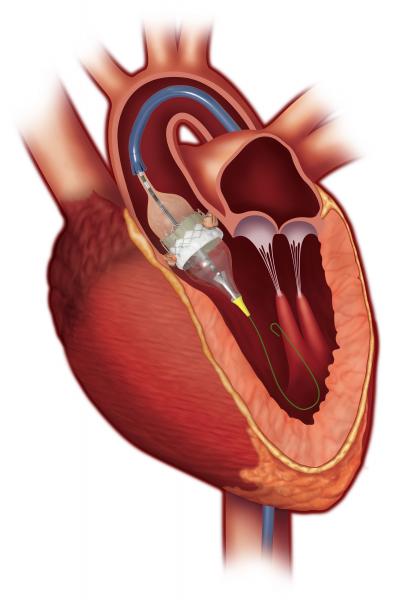
November 6, 2019 — Edwards Lifesciences announced it received European CE mark to expand use of the Edwards Sapien 3 ...

November 6, 2019 – The final, long-term, patient-level data for the Cook Medical Zilver PTX drug-eluting stent (DES) ...
November 6, 2019 – Positive, two-year data from the first-in-human study of Selution SLR, MedAlliance’s novel sirolimus ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
November 6, 2019 – Boston Scientific announced positive data for two of its devices within the peripheral drug-eluting ...
November 5, 2019 — The U.S. Food and Drug Administration (FDA) is updating its November 2017 safety communication to ...
November 5, 2019 — Philips Medical System is recalling the Forte Gamma Camera System due to the potential for the 660 ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
November 4, 2019 — Abbott is recalling its CentriMag Acute Circulatory Support System due to a calibration system error ...
My observation over the 13 years covering cardiology is that it is a heavily male-dominated field. This is more ...
The latest in interventional cardiology clinical data and new device technologies were highlighted at the annual Transcatheter Cardiovascular Therapeutics (TCT) conference. This is an overview of some of the biggest takeaways from TCT 2019, including short duration dual-antiplatelet therapy (DAPT) using newer generation DES; safety of paclitaxel coated devices; new TAVR devices seeking entry into the U.S. market; new COAPT Trial data on MitraClip use in heart failure patients; CT study of TAVR valve leaflet thrombosis; and potential game-changing technologies on the expo floor.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

November 1, 2019 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
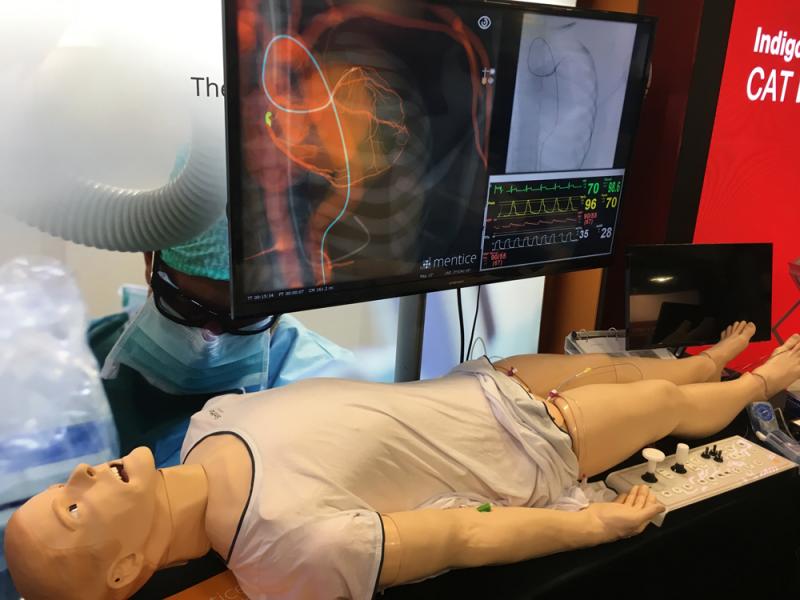
This is a photo essay of the interventional cardiology and structural heart technologies on the expo floor and discussed ...
October 30, 2019 – Impulse Dynamics received U.S. Food and Drug Administration (FDA) approval of a PMA supplement for ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
October 31, 2019 – In a large series of iliac vein stent cases, a blinded comparison found intravascular ultrasound ...
October 31, 2019 — AliveCor, a provider of smartphone based ECG technology and Huami Corp., a provider of biometric ...
October 31, 2019 — Baylis Medical announced the first North American use of its EPstar Fixed Electrophysiology Catheters ...

 November 06, 2019
November 06, 2019












