November 14, 2019 — More than a million Americans face a doubling in their risk of death during or while recovering from ...
November 14, 2019 — A new study from researchers at the Smidt Heart Institute at Cedars-Sinai shows that electronic ...
November 14, 2019 — Results were positive for the multicenter PROMISE I Trial represents the first human use in the ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
November 14, 2019 — A large majority of patients with atherosclerotic cardiovascular disease (ASCVD) in the NCDR ...
November 7, 2019 — The IN.PACT Arteriovenous (AV) Access randomized trial evaluating the safety and effectiveness of the ...

November 14, 2019 — The results of the 21 late-breaking clinical trials presented for the first time at Vascular ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
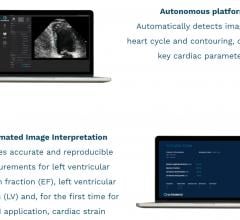
November 14, 2019 — Ultromics has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its ...
https://www.dicardiology.com/channel/pacemakersTexas Heart Institute (THI) was awarded a prestigious four-year, $2.39 million grant from the National Institutes of Health (NIH) to explore further the development of a novel pacemaker system that is both leadless and wirelessly powered.

This is a photo essay of new technologies and activities at the American Society of Nuclear Cardiology (ASNC) 2019 ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
November 7, 2019 — The REVEAL FDA investigational device exemption (IDE) trial confirmed a favorable safety and ...
November 7, 2019 — The Endospan Ltd. Nexus aortic arch stent graft is a CE mark–approved, off-the-shelf system for ...
November 11, 2019 — Although common femoral artery (CFA) endarterectomy is still considered the gold standard treatment ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
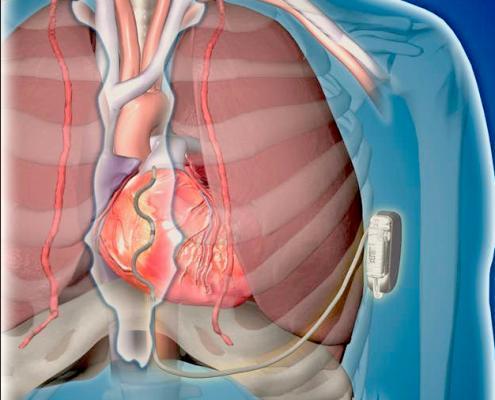
Cardiac rhythm management (CRM) devices in use today are evolving to raise the bar beyond monitoring and managing ...
November 11, 2019 — ECG Management Consultants, a leading U.S. healthcare advisory firm, is now part of the global ...
November 8, 2019 — Penumbra Inc. announced that the EXTRACT-PE trial successfully met the primary endpoints ...


 November 14, 2019
November 14, 2019