December 18, 2019 — In their latest report, “Cardiovascular Disease 2020-2030: Trends, Technologies & Outlook” IDTechEx ...
December 18, 2019 — Append Medical, developer of a novel left atrial appendage (LAA) closure device to minimize stroke ...
December 18, 2019 — BioVentrix, Inc., developer of the first less invasive system for left ventricular remodeling, today ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
December 18, 2019 — Cook Medical initiated a recall of its CrossCath Support Catheters in November, which the U.S. Food ...

December 16, 2019 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...

The U.S. Food and Drug Administration (FDA) approved the use of Vascepa (icosapent ethyl) capsules as an adjunctive therapy to reduce the risk of cardiovascular events in adults with elevated triglyceride levels
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
December 12, 2019 — Impulse Dynamics, developer of Optimizer Smart System for delivering CCM therapy, announced the ...
December 12, 2019 — Low-dose aspirin was not associated with a reduced risk of a fatal heart attack among African ...
December 12, 2019 — Cardiologs, a global leader in artificial intelligence (AI) cardiology diagnostics, announced today ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
December 9, 2019 — DiA Imaging Analysis Ltd., an IBM Alpha Zone Accelerator Alumni Startup, announces a collaboration ...
November 27, 2019 — CAE Healthcare will showcase its mixed reality training solutions for practicing physicians and ...
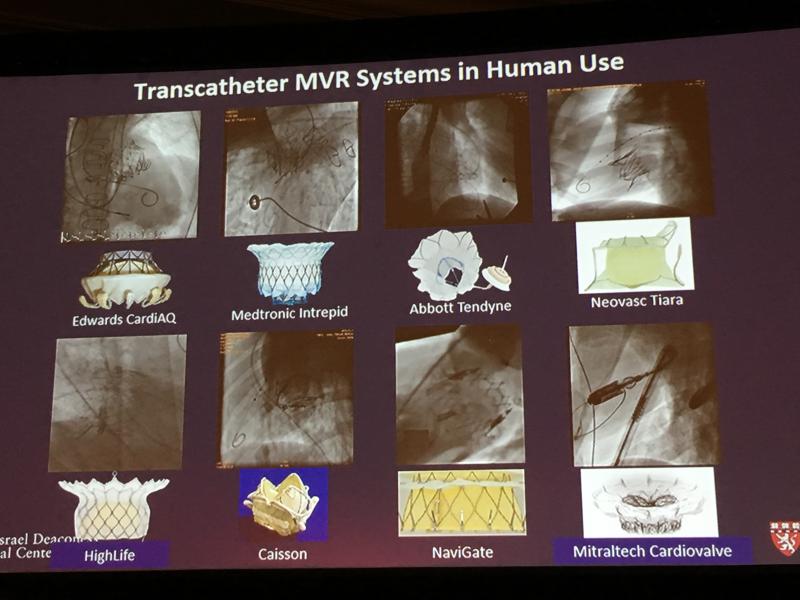
The overwhelming success story for transcatheter aortic valve replacement (TAVR) moving from a science project to ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

November 26, 2019 — Physicians from the University of New Mexico (UNM) and local emergency responders recently treated a ...
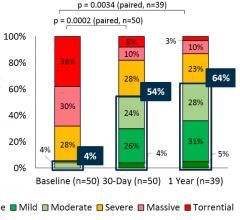
November 26, 2019 — The preliminary one-year results of the TRILUMINATE Pivotal Study for Abbott's TriClip device, a ...
November 26, 2019 — The University of Connecticut (UConn) Department of Kinesiology and Hartford Healthcare have ...

 December 18, 2019
December 18, 2019