June 12, 2019 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
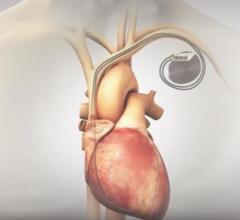
Boston Scientific Corp. has initiated the OPTION trial to compare safety and effectiveness of the next-generation Watchman FLX left atrial appendage closure (LAAC) platform to first-line oral anticoagulants (OAC) for stroke risk reduction in patients with non-valvular atrial fibrillation (AF) who undergo a cardiac ablation procedure. OACs used in the trial will include direct oral anticoagulants (DOAC) and warfarin.
Specialized risk scores derived from testing that calculates the cumulative effect of an individual’s entire DNA sequence may reliably predict heart disease in people who have not yet had a heart attack, according to new research. The research is published in Circulation: Genomic and Precision Medicine, an American Heart Association journal.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
In a letter sent to healthcare providers, the U.S. Food and Drug Administration (FDA) validates that Abiomed’s Impella RP heart pump is safe and effective for treatment of right heart failure. The letter comes after the FDA examined the results from Abiomed’s 18-month post-approval study (PAS) of 42 Impella RP patients. The data shows a 64 percent survival rate and 90 percent heart recovery for the subgroup of PAS patients who met the enrollment criteria of Impella RP’s premarket clinical studies. That survival rate is, as the FDA writes in its letter, “similar to the premarket clinical study survival rate,” which was 73 percent.
Centers for Medicare and Medicaid Services (CMS) Administrator Seema Verma addressed the American Medical Association (AMA) Annual Meeting of the House of Delegates, which ran June 8-12 in Chicago. In her remarks, Verma addressed current efforts by the Trump Administration to enact healthcare reform. The following is a transcript of her remarks.
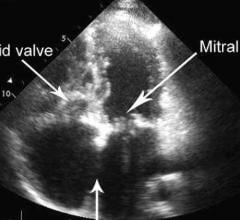
June 11, 2019 — Abbott recently announced positive late-breaking data from its TRILUMINATE study of the company's ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
W. L. Gore & Associates (Gore) announced the U.S. Food and Drug Administration’s (FDA’s) premarket approval (PMA) of the Gore Cardioform ASD Occluder for the percutaneous closure of ostium secundum atrial septal defects (ASDs). The FDA approval was supported by data collected from the pivotal stage of the Gore ASSURED Clinical Study, which demonstrated 100 percent closure success at the six-month evaluation in patients with a successful implant.
Medivis announced that its augmented reality (AR) technology platform for surgical applications, SurgicalAR, has received 510(k) clearance for clinical use in the operating room by the U.S. Food and Drug Administration (FDA). The New York City-based medical technology company will commence the immediate commercialization of the platform in the United States.
The Amsterdam University Medical Center has won MR Solutions’ Image of the Year 2019 award for the best molecular research image. Preclinical image submissions came in from most of MR Solutions’ users across the world. The winners, which were presented at the International Society for Magnetic Resonance in Medicine (ISMRM) conference, May 11-16 in Montreal, QC, Canada, were chosen by a panel of leading academics who examined images from magnetic resonance imaging (MRI), positron emission tomography (PET) or computed tomography (CT) – or a combination of imaging modalities.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Active duty Army personnel have worse cardiovascular health compared to people of similar ages in the civilian population, according to new research in Journal of the American Heart Association.
A discussion with Nicholas Bevins, Ph.D., vice chair, physics and research, and Jessica Harrington, RCIS. They explain ...
BGN Technologies, the technology transfer company of Ben-Gurion University (BGU), introduced a novel method for producing radioisotopes for nuclear medicine and medical imaging technologies such as computed tomography (CT) scan and positron emission tomography-computed tomography (PET-CT).
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
The Society of Cardiovascular Computed Tomography (SCCT) will present the 2019 Gold Medal Award to Jonathon Leipsic, M.D., FSCCT, and Gilbert Raff, M.D., FSCCT, at the SCCT 14th Annual Scientific Meeting, July 11 – 14 in Baltimore. The SCCT Gold Medal Award recognizes outstanding leaders who have made landmark contributions to the field of cardiovascular computed tomography and to the society.
Orchestra BioMed Inc. announced the presentation of two-year clinical data from the European Moderato I study of BackBeat Cardiac Neuromodulation Therapy (“BackBeat CNT”) for hypertension at the EuroPCR Conference, May 21-24 in Paris, France. Prof. Petr Neužil, M.D., Ph.D., CSc., FESC, head of the department of cardiology of Na Homolce Hospital in Prague, Czech Republic, presented data which showed a strong safety profile of BackBeat CNT and its ability to immediately, substantially and chronically lower blood pressure in patients with persistent hypertension (office BP > 150 mmHg) despite two or more anti-hypertensive medications and an indication for a pacemaker.
Royal DSM recently announced a collaboration with Strait Access Technologies (SAT), to develop the world’s first durable and cost-effective transcatheter replacement heart valves with polymeric leaflets.

 June 13, 2019
June 13, 2019