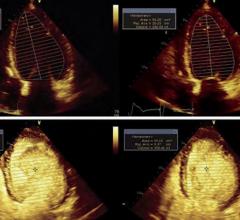
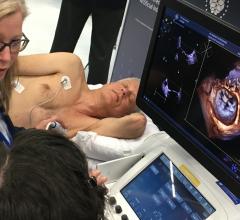
Biotronik announced the market release of its new injectable cardiac monitor (ICM), Biomonitor III, following approval in the CE region. The novel device is designed to help patients with irregular heart rhythms by documenting suspected arrhythmia or unexplained syncope with increased clarity.
Federico Asch, M.D., FASE, director of cardiac imaging research and director of the cardiovascular imaging lab, MedStar ...
Partho Sengupta, M.D., MBBS, chief of cardiology, West Virginia Heart and Vascular Institute, explains how wearable ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Sharon Mulvagh, M.D., FASE, FACC, FRCPC, professor of medicine, division of cardiology, Dalhousie University, Halifax ...
This is a quick example of how artificial intelligence (AI) is being integrated on the back end of cardiac ultrasound ...
Marielle Scherrer-Crosbie, M.D., Ph.D., director of echocardiography at the Hospital of the University of Pennsylvania ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Federico Asch, M.D., FASE, director of cardiac imaging research and director of the cardiovascular imaging lab, MedStar ...
Judy Hung, M.D., director of echocardiography, Division of Cardiology, Massachusetts General Hospital, Boston, explains ...
Roberto Lang, M.D., director of cardiac imaging at the University of Chicago, has been working with TomTec for the past ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
The U.S. Food and Drug Administration (FDA) issued the final guidance: Marketing Clearance of Diagnostic Ultrasound Systems and Transducers. This final guidance provides detailed recommendations for manufacturers seeking marketing clearance of diagnostic ultrasound systems and transducers.
Thomas Porter, M.D., FASE, the Theordore F. Hubbard Distinguished Chair of Cardiology and a professor of medicine at the ...
Siemens Healthineers and Mentice AB announced the collaboration to fully integrate Mentice’s VIST Virtual Patient into the Artis icono angiography system from Siemens Healthineers. The VIST Virtual Patient thus becomes a fully integrated simulation solution for the angio suite, according to the companies. The global partnership between the two companies will allow interventional radiologists, neuroradiologists, and cardiologists to perform vascular and cardiac interventions on a virtual patient inside the angio-suite.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
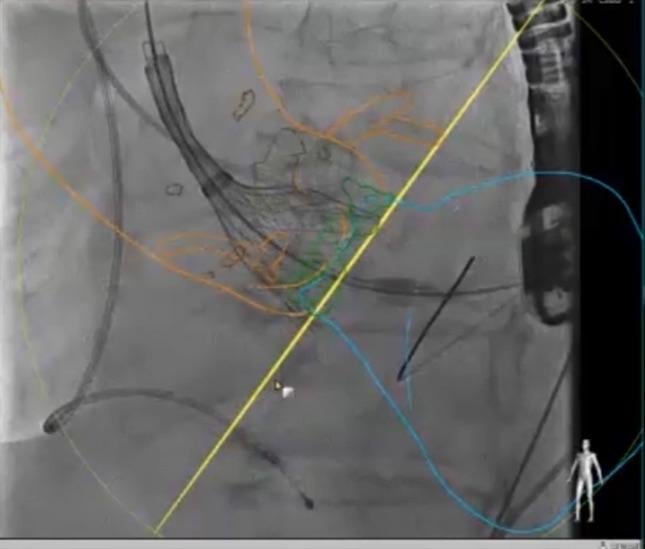
The Centers for Medicare and Medicaid Services (CMS) recently finalized its decision to update the national coverage policy for transcatheter aortic valve replacement (TAVR), streamlining key elements of the original national coverage determination that went into effect in 2012.
June 21, 2019 – Canon Medical Systems USA Inc. is showcasing its new line of ultrasound systems, the Aplio a-series, at ...
Jacques Kpodonu, M.D., FACC, cardiac surgeon, Beth Israel Deaconess Medical Center, and professor at Harvard Medical ...

 July 01, 2019
July 01, 2019