Arthur Agatston, M.D., clinical professor of medicine, Florida International University, Herbert Wertheim College of ...

One of the big trends in cardiac computed tomography (CT) imaging has been the introduction of noninvasive fractional ...
July 24, 2019 — The U.S. Food and Drug Administration announced Maquet/Datascope is recalling all intra-aortic balloon ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

July 24, 2019 — Atrial fibrillation (AFib) is a common abnormal heart rhythm. It is treated either with medications or ...
The West Virginia University (WVU) Heart and Vascular Institute is the first hospital in the country to acquire the Alphenix 4D CT from Canon Medical Systems USA Inc. The system offers an angiography configuration to expand capabilities in interventional procedures and help advance patient care in the community. The configuration pairs the Alphenix Sky + C-arm and Hybrid Catheterization Tilt/Cradle Table for interventional procedures with the Aquilion One/Genesis Edition computed tomography (CT) system, allowing clinicians to efficiently plan, treat and verify in a single clinical setting.
Cardiac amyloidosis is a highly morbid and underdiagnosed infiltrative cardiomyopathy that is characterized by the ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Medical device startup company Filterlex Medical recently completed a series A financing round, raising a total of $3 million for its Captis embolic protection device for reducing the risk of stroke and other complications during catheter-based structural heart procedures. Captis won Best Innovation Award at the EuroPCR 2019 innovation competition in Paris and was awarded a grant of $200,000 by the Jon DeHaan foundation.
A new method of evaluating irregular heartbeats outperformed the approach that’s currently used widely in stroke units to detect instances of atrial fibrillation (Afib).
Ron Blankstein, M.D., director of cardiac computed tomography, Brigham and Women's Hospital, and associate professor of ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Quynh Truong, M.D., MPH, associate professor of radiology and medicine at Weill Cornell and director of cardiac CT ...
Low doses of radiation equivalent to three computed tomography (CT) scans, which are considered safe, give cancer-capable cells a competitive advantage over normal cells in healthy tissue, scientists have discovered. Researchers at the Wellcome Sanger Institute and the University of Cambridge studied the effects of low doses of radiation in the esophagus of mice.
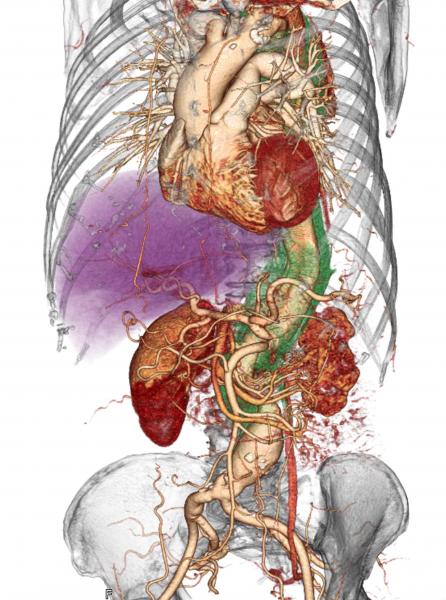
When a patient comes to the hospital with acute type A aortic dissection (ATAAD), a tearing of the inner lining of the aorta, surgeons typically perform an immediate open repair to prevent death from aortic rupture.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
On June 30, 2019, the Centers for Medicare & Medicaid Services (CMS) announced the Johns Hopkins University School of Medicine has been designated a qualified provider-led entity (qPLE). This allows Johns Hopkins to develop criteria that meet the requirements of the federal Protecting Access to Medicare Act (PAMA) of 2014 when ordering diagnostic imaging tests such as computed tomography (CT) scans, magnetic resonance imaging (MRI) scans and nuclear imaging in the emergency department and ambulatory settings.
A new analysis published by The Sage Group LLC concludes that the all-cause cost of critical limb ischemia (CLI) exceeds $200 billion in the United States.
The U.S. Food and Drug Administration (FDA) Center for Devices and Radiological Health (CDRH) announced the 2020 Experiential Learning Program site visit proposal solicitation period. The 2020 Fall ELP submission period is open from Monday July 8, 2019 – Thursday, August 8, 2019, at 12 p.m. EST. The ELP is intended to provide a formal training mechanism for regulatory review staff to visit research, clinical, manufacturing and healthcare facilities to observe how medical devices are designed, developed and utilized.


 July 24, 2019
July 24, 2019