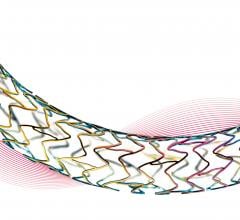
Biotronik's ultrathin Orsiro stent demonstrated superiority over Xience with respect to target lesion failure (TLF) at 12 months, according to newly released data from the BIOSTEMI trial.1 At the European Society of Cardiology (ESC) 2019 Congress, Aug. 31-Sept. 4 in Paris, France, Juan Fernando Iglesias, M.D., Ph.D., Geneva University Hospitals, Geneva, Switzerland, unveiled the results of the randomized controlled trial (RCT) in a late-breaking session. The results have also been published in The Lancet.
I recently had a great conversation with cardiac computed tomography (CT) pioneer Arthur Agatston, M.D., who is the ...
Abbott announced the launch of the company's TRILUMINATE Pivotal trial evaluating the safety and effectiveness of its TriClip transcatheter tricuspid valve repair system for the treatment of severe tricuspid regurgitation (TR). This is the first pivotal Investigational Device Exemption (IDE) trial in the U.S. to evaluate a catheter-based, non-surgical treatment for patients with severe TR – a condition in which the valve does not close properly, allowing blood to flow backward into the heart, which forces the heart to work harder. In severe cases, this condition can potentially lead to heart failure if left untreated.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
At the European Society of Cardiology (ESC) Congress 2019 together with the World Congress of Cardiology, the World Heart Federation launched a new “roadmap” aimed at reducing the global burden of CVD in people living with diabetes. The Roadmap on the prevention of cardiovascular disease among people living with diabetes is a key reference document for anyone involved in the planning, organization, implementation and monitoring, and evaluation of approaches related to CVD prevention in people living with diabetes. It outlines a vision of an ideal pathway of care, potential roadblocks along this pathway and proposed solutions, with examples from practice.
September 4, 2019 – Novartis announced results from two new clinical trials evaluating improvement in heart structure ...
September 4, 2019 — Final data from the World Alliance Societies of Echocardiography (WASE) Normal Values Study was ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Shockwave Medical Inc. has received Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) for its Shockwave intravascular lithotripsy (IVL) system with the Shockwave C2 Coronary IVL Catheter. The device is currently the subject of an Investigational Device Exemption (IDE) study called DISRUPT CAD III. The Shockwave C2 IVL Catheter is a proprietary tool designed to fracture problematic calcium using sonic pressure waves in order to facilitate stent delivery, deployment and optimal expansion, thereby improving blood flow to the heart muscle.
Corindus Vascular Robotics Inc. announced that EClinicalMedicine, a clinical journal published by The Lancet, has published the results from the Telerobotic Intervention Study, the world’s first percutaneous coronary intervention (PCI) procedures conducted from a remote location outside the catheterization lab. The study was conducted using Corindus’ CorPath technology platform.
New Zealand-based Adept Medical announced the launch of the Antegrade IR Platform. Clinically driven, it is placed to provide an ideal work surface for antegrade femoral approach during interventional radiology vascular procedures. The device sits within the company’s existing range of access and patient positioning devices designed for interventional radiology, cardiology and vascular procedures.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

September 3, 2019 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
Philips will showcase its latest cardiac care innovations at the European Society of Cardiology (ESC) Congress 2019, Aug. 31–Sept. 4 in Paris, France. At the congress, Philips is showcasing Release 5.0 of its Epiq CVx cardiology platform for the first time in Europe. The platform includes automated applications for 2-D assessment of the heart, as well as robust 3-D right ventricle volume and ejection fraction measurements, making accurate exams faster and easier to conduct. Philips also announced that it is collaborating with digital health company LindaCare to combine the latter’s OnePulse cloud-based solution for the remote monitoring of patients with cardiac implantable electronic devices (CIEDs) with the Philips IntelliSpace Cardiovascular informatics platform.

Until recently, cardiologists trying to diagnose and treat arrhythmias have had to deal with technological limitations ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Use of the Internet of Things (IoT) is booming, with IHS Markit forecasting there will be 73 billion connected devices in use around the world by 2025. IoT technology has moved beyond speakers and smart fridges and is increasingly being utilized for critical applications across the healthcare industry like insulin delivery devices, connected inhalers and even cancer treatments.
In the near future, doctors may be able to apply artificial intelligence (AI) to electrocardiogram data in order to measure overall health status, according to new research. The study was published in Circulation: Arrhythmia and Electrophysiology, a journal of the American Heart Association.
Atrial fibrillation, or Afib, kills about 130,000 people worldwide every year. It also affects 3 to 6 million people in ...

 September 05, 2019
September 05, 2019