Torsten Vahl, M.D., director of experimental and translational research, Structural Heart and Valve Center and at the ...
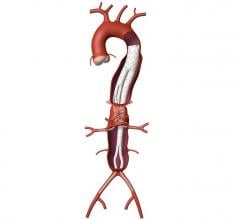
Medtronic plc announced it has received Breakthrough Device designation from the U.S. Food and Drug Administration (FDA) for its Valiant TAAA Stent Graft System for minimally invasive repair of thoracoabdominal aortic aneurysm (TAAA). A TAAA is a complex condition causing a bulging of the aorta, which extends from the chest down into the abdomen.
October 9, 2019 — Medtronic is recalling the 6 French Sherpa NX Active Guide Catheter due to a risk of the outer ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Douglas Drachman, M.D., FACC, has been selected as the next vice chair of the American College of Cardiology’s (ACC) Annual Scientific Session. Drachman will serve as vice chair for ACC.21 and ACC.22 and transition to chair for ACC.23 and ACC.24.
PQ Bypass Inc. announced it has received full approval of its investigational device exemption (IDE) trial of the company’s Torus stent graft. The novel stent graft platform is designed for the treatment of peripheral artery disease (PAD) in the superficial femoral artery (SFA). By acquiring early U.S. Food and Drug Administration (FDA) feedback through the pre-submission process, the TORUS-2 study (The PQ Bypass pivOtal IDE intra-aRterial stent graft study for occlUsive and re-Stenotic fem-pop revascularization) is the company’s second IDE approved in less than two years and is the first pivotal IDE for an SFA stent graft since Viabahn received initial premarket approval (PMA) in 2005.
American Heart Association President Robert Harrington, M.D., explains the reasons for shorter duration dual-antiplatelet therapy (DAPT) in high-risk bleeding patient
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
William O’Neill, M.D., medical director of the Center for Structural Heart Disease at Henry Ford Hospital, Detroit, explains data on first 250 patients in the National Cardiogenic Shock Initiative Study (NCSI) and new escalation protocols
Henry Ford Hospital in Detroit gets a lot of referrals for very sick patients seeking a last resort treatment in its ...

October 7, 2019 — Glynn Crawford was hospitalized at University Hospitals Ahuja Medical Center in late June/early July ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
October 7, 2019 – A recent study published in the New England Journal of Medicine supports the use of cardiac magnetic ...
Chandan Devireddy, M.D., offers insights about what he saw as the top take aways from the 2019 Transcatheter ...
October 4, 2019 – A new analysis of the PARTNER 3 Trial data found a modest, but significant, improvement in one-year ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
October 4, 2019 – Results of a new economic analysis of the COAPT Trial data found that transcatheter mitral valve ...
October 4, 2019 – RenalGuard Therapy was found to be superior to the POSEIDON method in preventing contrast-induced ...
October 3, 2019 – Five-year results from the PARTNER 2A Trial found patients with severe aortic stenosis (AS) and ...


 October 09, 2019
October 09, 2019