Royal Philips introduced two new balloons to its Stellarex 0.035-inch low-dose drug-coated balloon (DCB) portfolio. The new 200mm and 150mm Stellarex 0.035-inch low-dose DCBs have received approval from the U.S. Food and Drug Administration (FDA) for the treatment of de novo and restenotic lesions in native superficial femoral or popliteal arteries, both arteries in the upper leg. The new balloons broaden physicians’ treatment options for peripheral artery disease (PAD) patients with a high risk of restenosis. The 200mm and 150mm Stellarex 0.035-inch low-dose DCBs are now available in the U.S. and will be rolled out to other markets in due course.
On-the-job exposure to high levels of pesticides raised the risk of heart disease and stroke in a generally healthy group of Japanese American men in Hawaii, according to new research. The study was published in the Journal of the American Heart Association, the open access journal of the American Heart Association.
October 15, 2019 — Kardium Inc. announced that the Globe mapping and ablation system has successfully demonstrated ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The U.S. Food and Drug Administration (FDA) issued two final guidances for the performance testing and labeling of cardiac catheterization lab devices.
Medtronic plc announced the start of a worldwide pivotal study evaluating its investigational Extravascular Implantable Cardioverter Defibrillator (EV ICD) system to treat dangerously fast heart rhythms. The EV ICD system is designed to deliver lifesaving defibrillation and pacing therapy via a device the same size as traditional, transvenous ICDs, but with a lead (thin wire) placed outside the heart and veins.
October 11, 2019 — The ultra-thin Supraflex drug-eluting stent (DES) from SMT maintained numerically lower outcomes at ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

The Cardiovascular Research Foundation (CRF) has announced the 12 late-breaking trials and 16 late-breaking science presentations that will be reported at the Transcatheter Cardiovascular Therapeutics (TCT) 2019 scientific symposium. TCT, the world’s premier educational meeting specializing in interventional cardiovascular medicine, will take place Sept. 25 – 29, 2019 at The Moscone Center in San Francisco.
Ajay J. Kirtane, M.D., associate professor of medicine at Columbia University Irving Medical Center and director of the cardiac catheterization laboratories at NewYork-Presbyterian (NYP) Hospital, shares the findings of the late-breaking EVOLVE Short DAPT study presented as a late-breaking trial at the 2019 Transcatheter Cardiovascular Therapeutics (TCT) meeting.
October 10, 2019 — An independent analysis of Lutonix 035 drug-coated balloon (DCB) patient-level data showed no ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
October 10. 2019 — Late-breaking results from its MODERATO II double-blind, randomized study of BackBeat Cardiac ...
Roxana Mehran, M.D., FACC, FACP, FCCP, FESC, FAHA, FSCAI, professor of medicine and director of interventional ...
Fujifilm Medical Systems U.S.A. Inc. recently launched Synapse Cardiology PACS 5.6.1, the company’s next-generation server-side rendering solution to help streamline image review and reporting across cardiovascular modalities. This technology is currently in clinical use at North Memorial Health, a Minneapolis-area health system and one of the nation’s top-rated hospitals for cardiovascular care.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
In the complex and fast-paced world of healthcare, customers face the challenge of staying up-to-date with the most advanced systems while also keeping costs down. Providers now have a solution from Canon Medical Systems USA: the Alphenix Encore Plus Program. The program enables customers to upgrade their aging angiography systems to the latest technology while maintaining many existing parts, delivering clinical excellence, operational continuity and cost savings.
Acutus Medical and Innovative Health announced a partnership that will offer advanced electrophysiology technology to improve patient outcomes in a cost-efficient manner. The two high profile venture-backed companies will team to offer hospital customers a comprehensive suite of EP products, including Acutus’ next-generation EP mapping and accessory products together with a growing portfolio of reprocessed single-use EP devices from a range of manufacturers.
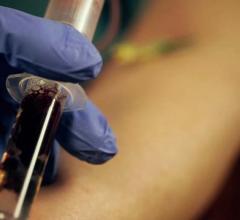
Abbott recently announced that its Architect Stat High Sensitivity Troponin-I blood test has received clearance from the U.S. Food and Drug Administration (FDA). As one of the most researched troponin diagnostic tests, doctors in the U.S. can now utilize this proven technology to help detect heart attacks faster and more accurately than contemporary troponin tests.


 October 15, 2019
October 15, 2019