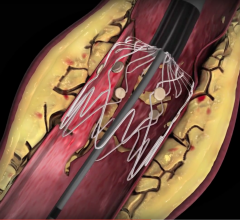
November 8, 2019 — Penumbra Inc. announced that the EXTRACT-PE trial successfully met the primary endpoints ...
November 8, 2019 — Dissection is a frequent and clinically problematic outcome of percutaneous transluminal balloon ...
November 8, 2019 — The Absorb bioresorbable stent (BVS) demonstrated good patency and clinical outcomes in patients with ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
American Society of Nuclear Cardiology (ASNC) President Rob Beanlands, M.D., shares a couple trends he sees in cardiac nuclear imaging.
Piotr Slomka explains how his team at Cedars-Sinai is working on intelligent patient risk prediction algorithms...
November 6, 2019 — Saranas Inc. announced completion of the first U.S. commercial case using its Early Bird Bleed ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
November 7, 2019 — Although the Abbott Absorb fully bioresorbable drug-eluting stent (DES) was taken off the market due ...
November 7, 2019 — Real-world, four-year study results of the In.Pact Admiral drug-coated balloon (DCB) demonstrate long ...
November 7, 2019 — There was no difference between drug-coated balloons (DCB) vs. plain old balloon angioplasty (POBA) ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
November 6, 2019 — Cleveland Clinic announced the Top 10 Medical Innovations for 2020 at a multimedia presentation last ...
Doctor Robert Hendel explains some of the new cardiac radiotracers in the pipeline that were discussed in sessions at the American Society of Nuclear Cardiology (ASNC) 2019 meeting...
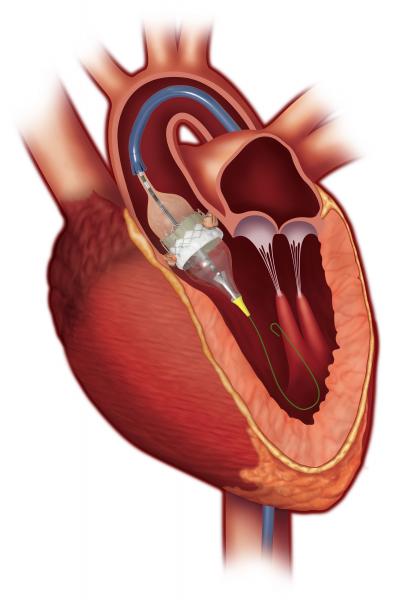
November 6, 2019 — Edwards Lifesciences announced it received European CE mark to expand use of the Edwards Sapien 3 ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...

November 6, 2019 – The final, long-term, patient-level data for the Cook Medical Zilver PTX drug-eluting stent (DES) ...
November 6, 2019 – Positive, two-year data from the first-in-human study of Selution SLR, MedAlliance’s novel sirolimus ...
November 6, 2019 – Boston Scientific announced positive data for two of its devices within the peripheral drug-eluting ...


 November 08, 2019
November 08, 2019