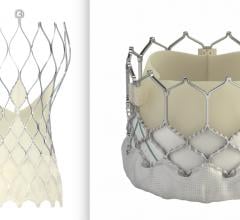
October 16, 2020 – Results from the XIENCE 90/28 clinical trials have shown that a shorter course of dual-antiplatelet ...
October 16, 2020 — The Restore EF Study demonstrates the use of contemporary best practices, including attempting a more ...
October 15, 2020 – The REFLECT II randomized clinical trial evaluating the safety and efficacy of the Keystone Heart ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
October 15, 2020 — The one-year results of the late-breaking DEFINE PCI study showed patients had improved outcomes and ...
October 13, 2020 — GE Healthcare announced U.S. FDA 510k clearance for its Ultra Edition package on Vivid cardiovascular ...
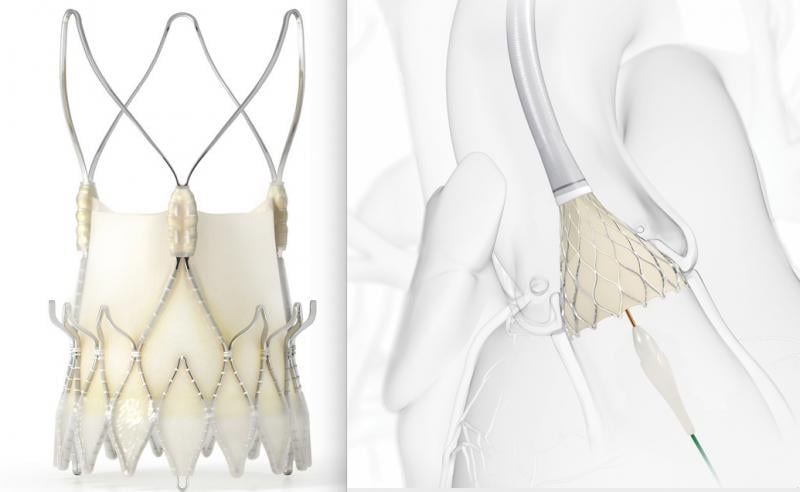
October 15, 2020 – The SCOPE II trial comparing the Boston Scientific Acurate neo vs. Medtronic CoreValve Evolut TAVR ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
October 15, 2020 – The MITHRAS randomized clinical trial found that interventional closure of an iatrogenic atrial ...

October 15, 2020 — The late-breaking studies are one of the hallmarks of the annual Transcatheter Cardiovascular ...
October 14, 2020 — American College of Cardiology (ACC) Quality Summit virtual meeting Oct. 8-9, 2020, featured several ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
October 14, 2020 — The American College of Cardiology (ACC) and the Cardiovascular Research Foundation (CRF) are ...
October 14, 2020 — Medtronic announced it is starting a randomized, head-to-head study comparing two transcatheter ...
October 14, 2020 – The first outcomes data from the North American COVID-19 ST-Segment Elevation Myocardial Infarction ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

This is an overview of some of the biggest cardiology technology advances. These innovations are covered in more detail ...

For all the changes in medicine there are some things that seem resolutely stable. Chief among these is the idea that ...
October 8, 2020 — Galaxy Medical, a developer of pulsed electric field (PEF) technology for the treatment of cardiac ...


 October 16, 2020
October 16, 2020