Interview with Mike Stone, M.D., an emergency physician at Northwest Acute Care Specialists in Portland, Ore., director ...
March 16, 2020 — The European Society of Cardiology (ESC) issued a statement March 13 recommending in novel coronavirus ...
March 16, 2020 — The Society of Cardiovascular Computed Tomography (SCCT) released a new expert consensus document on ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Interview with Navin Kapur, M.D., FAHA, FACC, FSCAI, executive director, The CardioVascular Center for Research and ...

Philips is working on a prototype cath lab angiographic imaging system that might be able to replace the current X-ray ...

Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
March 10, 2020 — The U.S. Food and Drug Administration (FDA) has cleared the SynCardia Systems 50cc temporary Total ...

March 10, 2020 — Novel coronavirus (COVID-19) has claimed another large cardiology meeting, the European Heart Rhythm ...

Cardiovascular disease is the leading cause of death for women in North America, and women with heart failure often ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

March 9, 2020 — The American College of Cardiology (ACC) is offering updates on the clinical impact of novel coronavirus ...
March 9, 2020 — As the novel coronavirus (COVID-19) slowly spreads across the globe, more industry conferences are ...

March 9, 2020 — Less than week after the American College of Cardiology (ACC) said it would push ahead with holding its ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
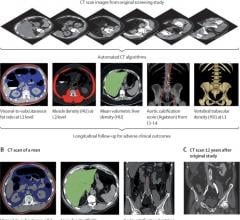
March 6, 2020 — Researchers at the National Institutes of Health and the University of Wisconsin have demonstrated that ...

March 5, 2020 – Due to the rapidly evolving novel coronavirus (COVID-19) situation in the U.S. and internationally, the ...
March 5, 2020 — The U.S. Food and Drug Administration (FDA) cleared the Acutus Medical SuperMap, an addition to its ...


 March 17, 2020
March 17, 2020