May 12, 2020 — The U.S. Food and Drug Administration (FDA) has cleared the next-generation CardioFocus Inc. HeartLight ...
May 12, 2020 — Medis acquired Advanced Medical Imaging Development S.r.l. (AMID), a developer and supplier specialized ...
May 11, 2020 — The American Society for Preventive Cardiology (ASPC) is collaborating with Intervent International to ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
May 11, 2020 – Competitive athletes are a rapidly growing population worldwide. Habitual vigorous exercise, a defining ...
May 11, 2020 — The American College of Cardiology (ACC) will begin collecting COVID-19 (SARS-CoV-2) data through the ...
May 8, 2020 — Final results from the UNTOUCHED study of the Emblem Subcutaneous Implantable Defibrillator (S-ICD) System ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
May 8, 2020 – A new clinical study found that patients with atrial fibrillation (AF) experienced a high incidence of ...
May 8, 2020 — Positive 12-month results were announced today from the PINNACLE FLX clinical trial assessing the safety ...
May 8, 2020 – Results from a first-in-human early feasibility study (EFS) using a saline enhanced radiofrequency (SERF) ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
May 8, 2020 — New clinical trial reveals the first-in-human results for paroxysmal or persistent atrial fibrillation (AF ...

May 8, 2020 — A new clinical trial is the first to compare the safety and efficacy of subcutaneous implantable ...
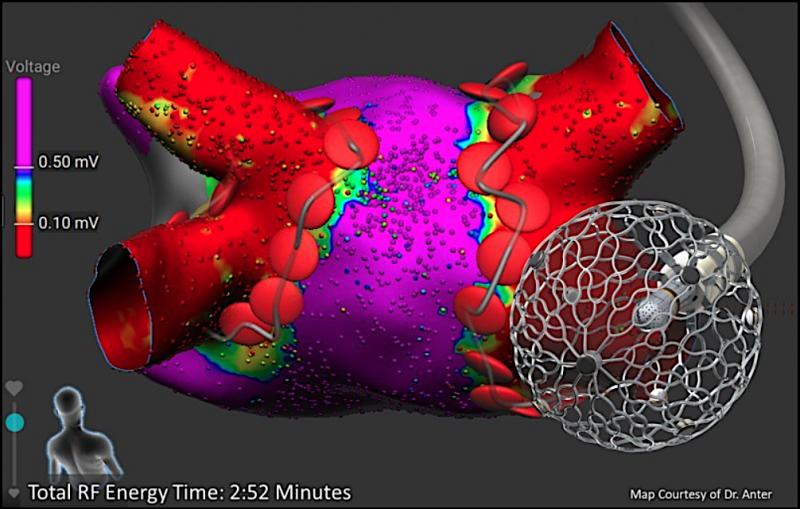
May 8, 2020 — A new clinical trial effectively uses pulsed field (PF) energy to treat patients with persistent or ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
May 2020 — An necklace has been developed that detects abnormal heart rhythm will be showcased for the first time on ...
Interview with Geoffrey Rose, M.D., president of Sanger Heart and Vascular Institute with Atrium Health, in Charlotte ...

May 6, 2020 — The U.S. Food and Drug Administration approved AstraZeneca’s dapagliflozin (Farxiga) oral tablets for ...

 May 12, 2020
May 12, 2020













