June 19, 2020 — SMT (Sahajanand Medical Technologies Pvt. Ltd) said it acquired of the structural heart medical device ...
June 19, 2020 — iVascular SLU announced the global launch of Essential Pro, a novel coronary artery drug-coated balloon ...
June 17, 2020 — MedAlliance announced its second CE mark approval for its Selution SLR 0.014 percutaneous transluminal ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
June 16, 2020 — The 36-month results from Veryan Medical’s MIMICS-2 study for the BioMimics 3D femoropopliteal stent ...
June 16, 2020 - Biotronik has today announced its commitment to giving physicians additional tools to pace in the His ...
June 16, 2020 – The Montreal Heart Institute (MHI) and Thermedical, a developer of thermal-ablation systems to treat ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

June 15, 2020 — The U.S. Food and Drug Administration (FDA) today revoked the emergency use authorization (EUA) that ...
Jay Mohan, D.O., RPVI, interventional cardiology fellow at William Beaumont Hospital, Royal Oak, Michigan, created this ...
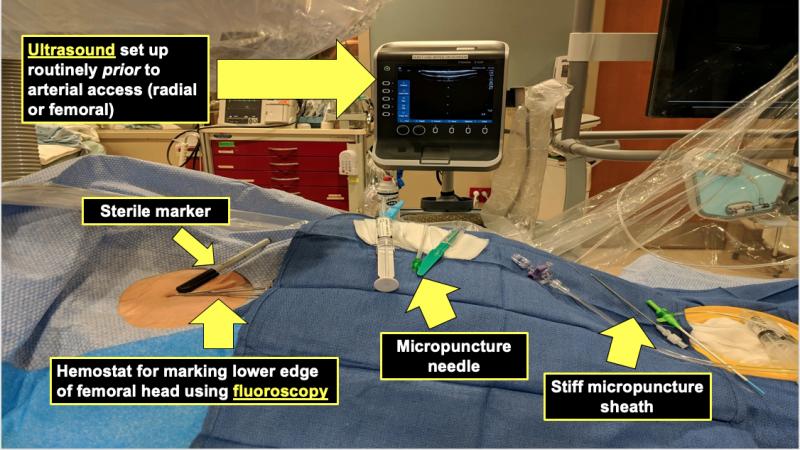
Vascular access site bleeding is associated with higher complications and mortality rates. For decades femoral access ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
The financial burden of COVID-19 has put tremendous pressure on health systems across the country. This webinar will ...
June 10, 2020 — In a new study, Johns Hopkins researchers found that testing people for SARS-CoV-2 (COVID-19) too early ...

June 9, 2020 — SMT (Sahajanand Medical Technology Private Ltd.), a leading medical device company in India focused on ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
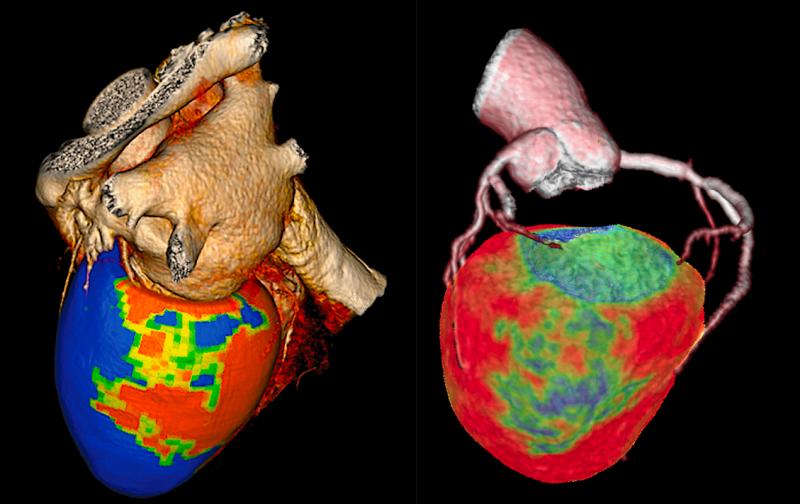
Here is an overview of a few of the biggest technology advances in cardiovascular computed tomography (CT). These are ...
June 8, 2020 — Edwards Lifesciences Corp. announced Chinese regulatory approval for the Edwards Sapien 3 transcatheter ...
June 8, 2020 – BD (Becton, Dickinson and Company) launched the Halo One Thin-Walled Guiding Sheath, designed to perform ...


 June 19, 2020
June 19, 2020









![Centricity Cardio Enterprise[1] is an integrated Cardiovascular PACS (CVPACS) and Information System (CVIS) that bridges the gaps between care areas and healthcare information systems. You get a single point of access for patient data, wave forms, images, analysis tools and physician reports – combined with powerful end-to-end management, analytics and workflow tools across the cardiovascular care pathway.](/sites/default/files/styles/content_feed_medium/public/GESplashImage.jpeg?itok=lyl7zdal)