June 23, 2020 — The U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Program status for the ...
Prior to January 2020 when clinicians read about the history of the 1918 flu, and epidemiologists predicted we were ...
June 19, 2020 — SMT (Sahajanand Medical Technologies Pvt. Ltd) said it acquired of the structural heart medical device ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
June 19, 2020 — iVascular SLU announced the global launch of Essential Pro, a novel coronary artery drug-coated balloon ...
June 17, 2020 — MedAlliance announced its second CE mark approval for its Selution SLR 0.014 percutaneous transluminal ...
June 16, 2020 — The 36-month results from Veryan Medical’s MIMICS-2 study for the BioMimics 3D femoropopliteal stent ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
June 16, 2020 - Biotronik has today announced its commitment to giving physicians additional tools to pace in the His ...
June 16, 2020 – The Montreal Heart Institute (MHI) and Thermedical, a developer of thermal-ablation systems to treat ...

June 15, 2020 — The U.S. Food and Drug Administration (FDA) today revoked the emergency use authorization (EUA) that ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Jay Mohan, D.O., RPVI, interventional cardiology fellow at William Beaumont Hospital, Royal Oak, Michigan, created this ...
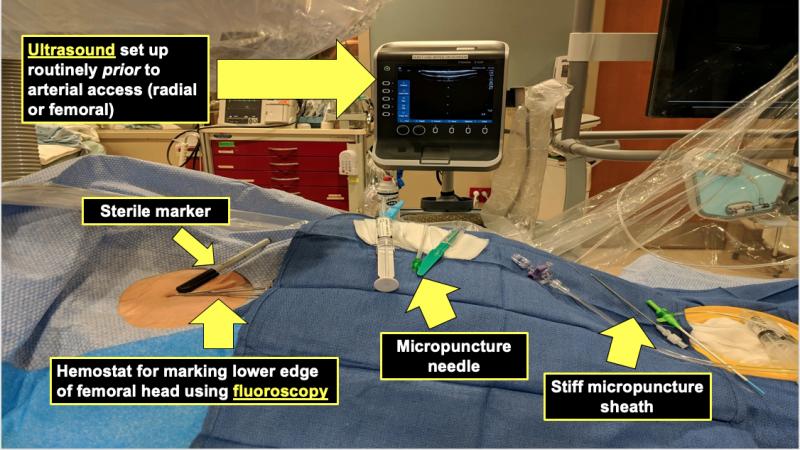
Vascular access site bleeding is associated with higher complications and mortality rates. For decades femoral access ...
The financial burden of COVID-19 has put tremendous pressure on health systems across the country. This webinar will ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
June 10, 2020 — In a new study, Johns Hopkins researchers found that testing people for SARS-CoV-2 (COVID-19) too early ...

June 9, 2020 — SMT (Sahajanand Medical Technology Private Ltd.), a leading medical device company in India focused on ...
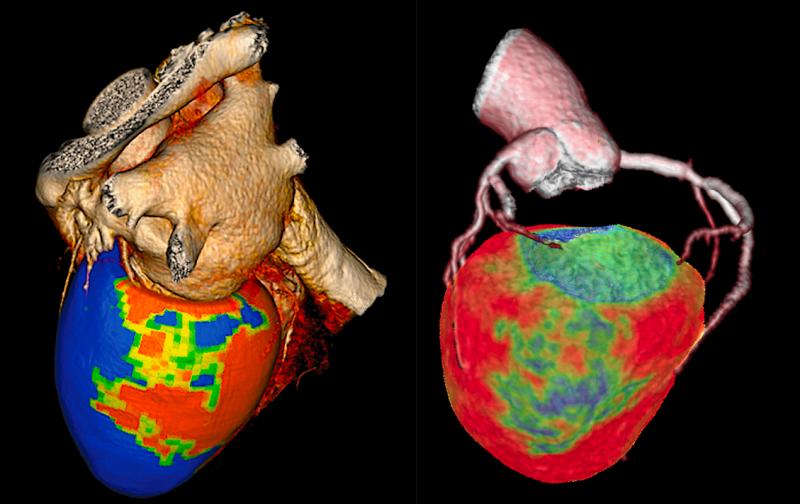
Here is an overview of a few of the biggest technology advances in cardiovascular computed tomography (CT). These are ...


 June 23, 2020
June 23, 2020











![Centricity Cardio Enterprise[1] is an integrated Cardiovascular PACS (CVPACS) and Information System (CVIS) that bridges the gaps between care areas and healthcare information systems. You get a single point of access for patient data, wave forms, images, analysis tools and physician reports – combined with powerful end-to-end management, analytics and workflow tools across the cardiovascular care pathway.](/sites/default/files/styles/content_feed_medium/public/GESplashImage.jpeg?itok=lyl7zdal)

