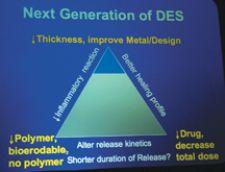
Examples of physicians’ declining use of drug-eluting stents are growing. According to Dr. Louis Cannon, Cardiac & ...
Arrow’s Transradial introducers provide for durable radial artery access while being vessel friendly. Our newest kit contains our .018 micro-puncture wire guide and needle set to minimize vessel trauma. The 6 French sheath and dilator track well over the smaller wire and we’ve applied a hydrophilic coating on the polyurethane sheath to enhance insertion and removal.
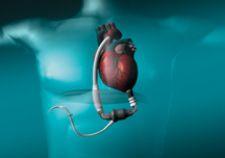
In the simplest of terms, a ventricular assist device (VAD) is a battery-operated mechanical pump that helps a weakened ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

Technological trendsetters within the cardiology arena have been embarking on a path of seamless integration of their ...
In the world of cardiologists who treat adults with congenital heart disease, the unusual is not so very unusual and the rare is fairly commonplace. Take Wendy Book, M.D., for example — this week she might meet for the first time a patient presenting with a heart defect from birth for which there is no standard therapy, and for whom there may well be no exact replica on record.
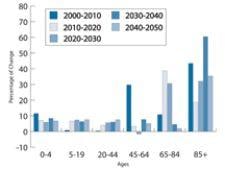
In 2006, the baby boom generation (those born from 1946 - 1964) started turning 60 at the rate of 330 per hour ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
In a giant leap forward in ICD patient safety, the American College of Cardiology Foundation (ACCF), in conjunction with the Heart Rhythm Society (HRS), established the National Cardiovascular Data Registry's national ICD Registry in the fall of 2005 to measure ICD patient outcomes and track patient trends.

Sometimes it's the smallest things that leave the strongest impression. Of the dozens of technologies with which I came ...
May 7, 2007 Stereotaxis Inc. announced today that it has developed an innovative new single-screen user interface that ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
May 7, 2007 — Ortho-Clinical Diagnostics Inc. has initiated a voluntary, nationwide recall of two lots of a diagnostic ...
May 7, 2007 — Signalife Inc. has announced that it has submitted its Fidelity 200 Cardiac Event Recorder 510K pre-market ...
May 7, 2007 — Thoratec Corp., a global maker of products to treat cardiovascular disease, says the FDA has approved an ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
May 7, 2007 — Steven E. Nissen, M.D., chairman of the Department of Cardiovascular Medicine at Cleveland Clinic, and immediate past president of the American College of Cardiology, has been named one of the 100 Most Influential People by Time magazine. He will be featured among other "Scientists and Thinkers."
May 7, 2007 — Australia's adult stem cell company, Mesoblast Limited, has announced that the FDA has cleared the Investigational New Drug Submission (IND) of its U.S.-based sister company, Angioblast Systems Inc., to commence a Phase 2 clinical trial of its allogeneic, or 'off-the-shelf,’ adult stem cells for patients with heart attacks.
May 7, 2007 — Citing issues related to potentially suboptimal therapeutic dosing of paclitaxel, Conor Medsystems, LLC ...

 May 07, 2007
May 07, 2007





