August 21, 2007 - Only half of adult women have had their cholesterol tested in the past year, according to the results ...
August 21, 2007 - Research conducted at the University of Texas Southwestern Medical Center in Dallas and appearing in ...
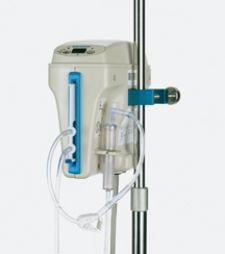
Induced hypothermia has traditionally been used perioperatively as a strategy for neurological protection during major vascular surgery or for situations where neurological injury is present or imminent. However, patients cannot deviate off of the normal core body temperature of around 37°C for long without risk of deleterious effects.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
August 20, 2007 - Cardiatis, SA announced that it has completed recruitment of patients into its first European clinical trial of its 3D Multilayer Braided Stent for repairing aneurysms.
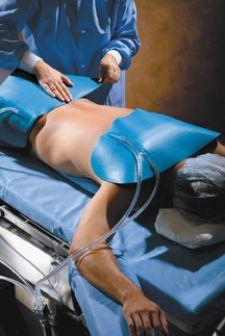
Controlling normothermia in perioperative patients can be almost as important to their health as the surgical procedure itself. Research is revealing more and more the negative implications of even small dips in patient temperature.

In the years since 9/11, disaster medicine has come into its own. Now a recognized specialty, the practice of disaster ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

Ice packs and cold compresses have been used for centuries to reduce swelling and fevers. But it’s only been within the ...
August 15, 2007 — ProSolv CardioVascular, a FUJIFILM company, announced that it is the newest MedAxiom Corporate Partner.
August 15, 2007 - InspireMD announced today receipt of orders for its MGuard Coronary stents from key markets in Western ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
August 14, 2007 — ProSolv CardioVascular, a FUJIFILM company, announced today that it is the newest MedAxiom corporate ...
August 15, 2007 - Thoratec Corp., a world leader in products to treat cardiovascular disease, said today that its PMA (PreMarket Application) seeking approval of its HeartMate II for bridge-to-transplantation will undergo review by an FDA advisory panel later this year.
August 15, 2007 - Merit Medical Systems Inc., a leading manufacturer and marketer of proprietary disposable devices used ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
August 15, 2007 - Performing cardiac CTA after coronary artery bypass surgery (CABG) can reveal unsuspected and ...
August 14, 2007 - Baseline serum complement component C4 is among independent predictors of stroke in patients with known or suspected coronary artery disease.
August 14, 2007 - ELA Medical Inc., a Sorin Group company that specializes in the design and manufacture of implantable ...

 August 20, 2007
August 20, 2007




