August 21, 2007 - Research conducted at the University of Texas Southwestern Medical Center in Dallas and appearing in ...
August 21, 2007 - Researchers at Rensselaer Polytechnic Institute have developed a new energy storage device that could ...
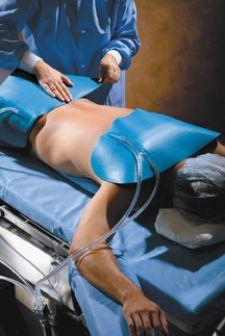
Controlling normothermia in perioperative patients can be almost as important to their health as the surgical procedure itself. Research is revealing more and more the negative implications of even small dips in patient temperature.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
August 20, 2007 - Cardiatis, SA announced that it has completed recruitment of patients into its first European clinical trial of its 3D Multilayer Braided Stent for repairing aneurysms.

In the years since 9/11, disaster medicine has come into its own. Now a recognized specialty, the practice of disaster ...

Ice packs and cold compresses have been used for centuries to reduce swelling and fevers. But it’s only been within the ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
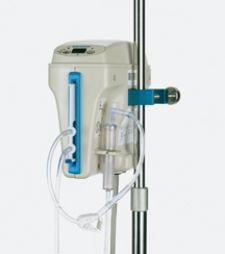
Induced hypothermia has traditionally been used perioperatively as a strategy for neurological protection during major vascular surgery or for situations where neurological injury is present or imminent. However, patients cannot deviate off of the normal core body temperature of around 37°C for long without risk of deleterious effects.
August 15, 2007 - InspireMD announced today receipt of orders for its MGuard Coronary stents from key markets in Western ...
August 14, 2007 — ProSolv CardioVascular, a FUJIFILM company, announced today that it is the newest MedAxiom corporate ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
August 15, 2007 - Thoratec Corp., a world leader in products to treat cardiovascular disease, said today that its PMA (PreMarket Application) seeking approval of its HeartMate II for bridge-to-transplantation will undergo review by an FDA advisory panel later this year.
August 15, 2007 - Merit Medical Systems Inc., a leading manufacturer and marketer of proprietary disposable devices used ...
August 15, 2007 - Performing cardiac CTA after coronary artery bypass surgery (CABG) can reveal unsuspected and ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
August 15, 2007 — ProSolv CardioVascular, a FUJIFILM company, announced that it is the newest MedAxiom Corporate Partner.
August 14, 2007 - Baseline serum complement component C4 is among independent predictors of stroke in patients with known or suspected coronary artery disease.
August 14, 2007 - ELA Medical Inc., a Sorin Group company that specializes in the design and manufacture of implantable ...

 August 20, 2007
August 20, 2007




