
The battle for infection control is fought daily in wound management where the body's first line of defense against microbes has been breeched. Sometimes something old is made new again with a twist of new technology, as is the case in new infection-fighting wound care products that use shrimp shells, honey and silver as their key ingredients. The silver bullet
Monitoring system maker Mennen Medical recently said an additional monitor, the VitaLogik 4000 Series, will soon be ...
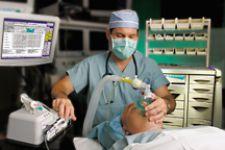
An Achilles' heal in the armor of hospital patient safety protocol is the fact that anesthesiologists are the only ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
InfoLogix Inc. will launch the SurgiChip, a real-time wireless solution designed to address leading causes of surgical ...

Many clinicians probably feel like they are being treated like a 5-year-old with the constant warnings and reminders to ...
April 9, 2008 - Michigan Instruments Inc. recently launched its new life support device, Life-Stat, that provides ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
RES-Q Healthcare Systems demonstrated its Patient Waiting Room View at AONE 2008. Similar to airline flight displays at ...

Antibiotics were a miracle drug when introduced more than 60 years ago, but they have also spurred the proliferation of antibiotic-resistant strains of super bug bacteria that hospitals are having a tough time controlling.
April 8, 2008 - Doctors at Boston Medical Center (BMC) led by Chief of Cardiac Surgery Robert Poston, M.D., have been ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
April 8, 2008 - People who are taking three or more drugs and still have high blood pressure have a condition known as ...
April 8, 2008 - Atrial fibrillation demonstrated that a port access, paracardioscopic Ex-Maze procedure produced favorable outcomes and allowed patients to discontinue antiarrhythmic drugs, according to interim results from a study presented by Andy C. Kiser, M.D., at the American College of Cardiology meeting in Chicago, IL.
MEDRAD Inc. won a Medical Design Excellence Award (MDEA) for the MEDRAD XDS Extravasation Detector, a device for the ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The Deficit Reduction Act of 2005 (DRA) has left its mark on hospitals and outpatient imaging center operators. There is no denying that the imaging industry has changed forever. Some may think that the 10 percent of operators on the brink of bankruptcy or in default on payments to finance vendors is a mere market correction for years of overgrowth. But do the math.
April 8, 2008 - The first-in-man study funded by MIV Therapeutics Inc. (MIV) showed that MIV's VESTAsync stents ...
April 8, 2008 - Battelle, a nonprofit independent research and development organization, and MEDRAD Inc. won a Medical ...

 April 08, 2008
April 08, 2008








