
(Editor's note: this article was updated in March 2017 with links at the bottom to more current stent technology ...

Physicians recognize the importance of ventricular assist devices (VADs) every time a congestive heart failure (CHF) ...
July 10, 2008 - Boston Scientific Corp. this week launched its Atlantis .018 Peripheral Imaging Catheter, which reportedly brings existing 40 MHz coronary standard ultrasound imaging resolution to peripheral interventions. The company said the product is available for immediately release in the U.S.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
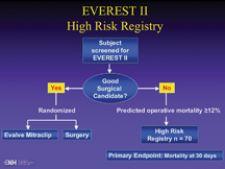
Preliminary results of a multicenter study show that the use of tiny clips to repair the mitral valve may someday ...
July 10, 2008 - Datascope Corp. said today it exercised its option to acquire the Peripheral Vascular Stent business of ...
The only safe assumption to make about today’s current gold standard imaging modality used to identify and evaluate atheromatous plaque in the peripheral arteries is that there is not one gold standard. To locate and evaluate these plaques that cause peripheral vascular disease (PVD), physicians have multiple tried-and-true imaging tests at their disposal.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
July 10, 2008 - A novel blood pool magnetic resonance angiography (MRA) agent, Vasovist (gadofosveset trisodium), has ...

Safe delivery of the balloon or stent through fragile vasculature during percutaneous transluminal coronary angioplasty (PTCA) procedures is an important criterion for successful patient outcomes.
July 9, 2008 – The Medical Imaging & Technology Alliance (MITA) today applauded the U.S. Congress for passing Medicare ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Siemens' ACUSON SC2000 volume imaging ultrasound system acquires nonstitched, real-time full-volume 3D images of the heart in one single heart cycle. Called “Echo in a Heartbeat,” this new technology generates nonstitched full-volume imaging reportedly of the entire heart with 90 degree pyramids and in just one second.
Angiotech Pharmaceuticals Inc. received 510(k) clearance from the FDA to market its 5-Fluorouracil (5-FU) coated central venous catheter (CVC).
July 9, 2008 - InnerSpace has added the V-Series line of heavy-duty cabinets, carts and workstations to its organized ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The FDA cleared Boston Scientific’s ALTRUA family of pacemakers, which enables the company to introduce its first Boston Scientific-branded pacemaker to treat bradycardia.
Midmark Diagnostics Group’s (MDG) IQmark Advanced Holter is designed to help physicians improve the efficiency, workflow and quality of care. Its reportedly comprehensive functionality offers users the option to control the parameters of the analysis, edit results, select report functions and provides quick and easy access to the entire full disclosure.
July 9, 2008 - New research shows catheter cryoablation is comparably effective to radiofrequency catheter ablation for the treatment of atrial flutter (AFL) and may have some safety advantages over the use of radiofrequency.

 July 09, 2008
July 09, 2008






