August 16, 2021 — The U.S. Food and Drug Administration (FDA) approved Abbott's Amplatzer Amulet Left Atrial Appendage ...
The in-person 2021 Healthcare Information Management Systems Society (HIMSS) meeting planned for Aug. 9-13 appeared to ...
August 9, 2021 — Boston Scientific Corp. has commenced enrollment in the HI-PEITHO clinical trial, a collaborative ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
August 9, 2021 - In association with Heart Rhythm 2021, Biotronik today announced that the latest implantable cardiac ...
August 9, 2021 – Results from an international clinical trial show a significant reduction in risk of ventricular ...
August 9, 2021 — Adagio Medical Inc., a provider of catheter ablation technologies for atrial fibrillation (AF) and ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

July 28, 2021 — Here is the complete list of late-breaking clinical trials (LBCT) and links to articles on all of them ...
August 9, 2021 — Boston Scientific is recalling INGENIO family of pacemakers and CRT-Ps due to the risk of incorrect ...
August 5, 2021 — A prospective, non-randomized, multicenter, first-in-human clinical study found good diagnostic and ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
August 5, 2021 — The U.S. Food and Drug Administration (FDA) has cleared clearance Abbott's latest optical coherence ...
August 3, 2021 — A subgroup analysis of the EAST – AFNET 4 study population revealed early initiation of rhythm control ...
August 4, 2021 — Temporary Spinal cord stimulation (SCS) was effective in suppressing post-operative atrial fibrillation ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
August 3, 2021 – Results from a new clinical trial show an increased risk of cardiac events, like sudden cardiac death ...
August 3, 2021 – A recent study unveiled a novel sinus node ablation technique that provides a safe and effective ...
August 3, 2021 – A new analysis of the Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA ...

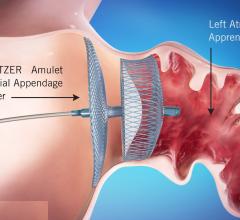
 August 16, 2021
August 16, 2021

















