November 12, 2021 — Orchestra BioMed Inc. announced multiple presentations of long-term clinical results and ISH ...

November 12, 2021 — President Joe Biden today announced his intention to nominate cardiologist Robert Califf, M.D., MACC ...
November 10, 2021 — Medtronic presented early data for its self-expanding Intrepid transcatheter mitral valve ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
November 10, 2021 — eMurmur, a technology partner to the global healthcare community, announces the world’s first open ...
November 10, 2021 — Biosensors International Group Ltd., a developer and manufacturer of innovative medical devices ...
November 10, 2021 — Shockwave Medical a pioneer in the development of Intravascular Lithotripsy (IVL) to treat severely ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
November 10, 2021 — Abbott released new global market research from its Beyond Intervention initiative, the company’s ...
November 10, 2021 — Shockwave Medical, a developer of Intravascular Lithotripsy (IVL) to treat complex calcified ...
November 10, 2021 — Ancora Heart Inc., a company developing a novel therapy to address heart failure, announced results ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
November 10, 2021 — Philips Healthcare announced North American availability of new innovations in its portfolio of ...
November 10, 2021 — Philips, a global leader in health technology, announced that it has signed an agreement to acquire ...
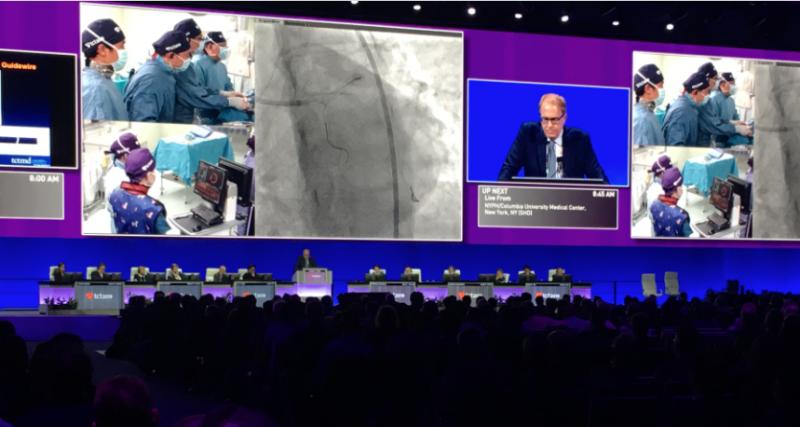
November 10, 2021 — There were nine late-breaking trials and 13 late-breaking science presentations at the 2021 ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
November 9, 2021 — Results from the largest randomized trial available comparing different closure device strategies ...
November 9, 2021 — Utilizing a magnetically-controlled capsule endoscopy system, the double-blind, randomized OPT-PEACE ...
November 9, 2021 — Six-month outcomes from the randomized RADIANCE-HTN TRIO Trial comparing endovascular ultrasound ...


 November 12, 2021
November 12, 2021